Analysis of Cold-Developed vs. Cold-Acclimated Leaves Reveals Various Strategies of Cold Acclimation of Field Pea Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.1.1. Plant Cultivation
2.2. Phenotyping Measurement/Evaluation
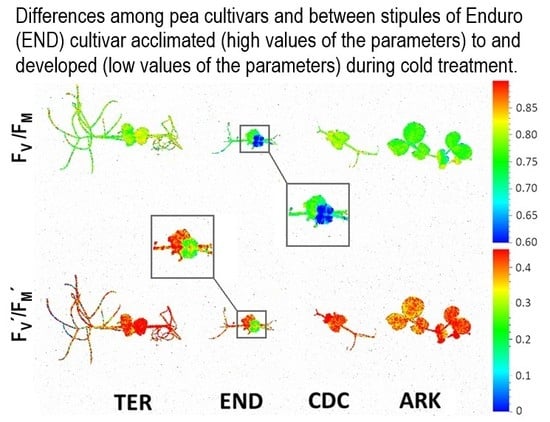
2.2.1. Chlorophyll Fluorescence Quenching Analysis
2.2.2. RGB Image Analysis
2.3. Chlorophyll Content and Chlorophyll Fluorescence Induction by Conventional Devices
2.4. Statistical Analysis
3. Results
3.1. Growth and Chlorophyll Content
3.2. Photosynthetic Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World; Oxford University Press: Oxford, UK, 2000; 316p. [Google Scholar]
- Riehl, S.; Zeidi, M.; Conard, N.J. Emergence of agriculture in the foothills of the Zagros Mountains of Iran. Science 2013, 341, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108 (Suppl. S1), S3–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biddle, A.J. Peas and beans. In Crop Production Science in Horticulture 25; CABI: Boston, MA, USA, 2017; pp. 3–9. [Google Scholar]
- Avia, K.; Pilet-Nayel, M.L.; Bahrman, N.; Baranger, A.; Delbreil, B.; Fontaine, V.; Hamon, C.; Hanocq, E.; Niarquin, M.; Sellier, H.; et al. Genetic variability and QTL mapping of freezing tolerance and related traits in Medicago truncatula. Theor. Appl. Genet. 2013, 126, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, T.; Pearce, S.P.; Stockinger, E.J.; Distelfeld, A.; Li, C.X.; Knox, A.K.; Vashegyi, I.; Vagujfalvi, A.; Galiba, G.; Dubcovsky, J. Regulation of freezing tolerance and flowering in temperate cereals: The VRN-1 connection. Plant Physiol. 2010, 153, 1846–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, M.D.; Thomashow, M.F. A role for circadian evening elements in cold-regulated gene expression in Arabidopsis. Plant J. 2009, 60, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Whitelam, G.C. Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat. Genet. 2007, 39, 1410–1413. [Google Scholar] [CrossRef]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [Green Version]
- Stoddard, F.L.; Balko, C.; Erskine, W.; Khan, H.R.; Link, W.; Sarker, A. Screening techniques and sources of resistance to abiotic stresses in cool-season food legumes. Euphytica 2006, 147, 167–186. [Google Scholar] [CrossRef]
- Mikić, A.; Mihailović, V.; Ćupina, B.; Đordević, V.; Milić, D.; Duc, G.; Stoddard, F.L.; Lejeune-Hénaut, I.; Marget, P.; Hanocq, E. Achievements in breeding autumn-sown annual legumes for temperate regions with emphasis on the continental Balkans. Euphytica 2001, 180, 57–67. [Google Scholar] [CrossRef]
- McPhee, K.E.; Muehlbauer, F.J. Registration of ‘Specter’ winter feed pea. J. Plant. Reg. 2007, 1, 118–119. [Google Scholar] [CrossRef]
- Xiaoyan, Z.; Shuwei, W.; Junjie, H.; Jinguo, H.; Tao, Y.; Xuxiao, Z. Large-scale evaluation of pea (Pisum sativum L.) germplasm for cold tolerance in the field during winter in Qingdao. Crop J. 2016, 5, 377–383. [Google Scholar]
- Lejeune-Henaut, I.; Hanocq, E.; Bethencourt, L.; Fontaine, V.; Delbreil, B.; Morin, J.; Petit, A.; Devaux, R.; Boilleau, M.; Stempniak, J.-J.; et al. The flowering locus Hr colocalizes with a major QTL affecting winter frost tolerance in Pisum sativum L. Theor. Appl. Genet. 2008, 116, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- National List of Varieties Listed in the State Variety Book by 15 June 2018; Central Institute for Supervising and Testing in Agriculture: Brno, Czech, 2018; 90p. (In Czech)
- Humplík, J.F.; Lazár, L.; Husičková, A.; Spíchal, L. Automated phenotyping of plant shoots using imaging methods for analysis of plant stress responses—A review. Plant Methods 2015, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enders, T.A.; St. Dennis, S.; Oakland, J.; Callen, S.T.; Gehan, M.A.; Miller, N.D.; Spalding, E.P.; Springer, N.M.; Hirsch, C.D. Classifying cold-stress responses of inbred maize seedlings using RGB imaging. Plant Direct 2019, 3, e00104. [Google Scholar] [CrossRef] [Green Version]
- Chiluwal, A.; Bheemanahalli, R.; Perumal, R.; Asebedo, A.R.; Bashir, E.; Lamsal, A.; Sebela, D.; Shetty, N.J.; Krishna Jagadish, S.V. Integrated aerial and destructive phenotyping differentiates chilling stress tolerance during early seedling growth in sorghum. Field Crop Res. 2018, 227, 1–10. [Google Scholar] [CrossRef]
- Lazár, D. Chlorophyll a fluorescence induction. Biochim. Biophys. Acta 1999, 1412, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Moura, D.S.; Brito, G.G.; Moraes, Í.L.; Fagundes, P.R.R.; Castro, A.P.; Deuner, S. Cold Tolerance in rice plants: Phenotyping procedures for physiological breeding. J. Agric. Sci. 2018, 10, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Hyldgaard, B.; Yu, X.; Rosenqvist, E.; Ugarte, R.M.; Yu, S.; Wu, Z.; Ottosen, C.-O.; Zhao, T. Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 2018, 214, 68. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Baldassarre, V.; Cabassi, G.; Ferrante, A. Use of chlorophyll a fluorescence for evaluating the quality of leafy vegetables. Aust. J. Crop Sci. 2011, 5, 735–741. [Google Scholar]
- Adamski, J.M.; Cargnelutti, D.; Sperotto, R.A.; Terra, T.F.; Rosa, L.M.G.; Cruz, R.P.; Fett, J.P. Identification and physiological characterization of two sister lines of indica rice (Oryza sativa L.) with contrasting levels of cold tolerance. Can. J. Plant Sci. 2016, 96, 197–214. [Google Scholar] [CrossRef] [Green Version]
- Warkentin, T.; Klassen, E.; Bing, D.; Lopetinsky, K.; Kostiuk, J.; Barlow, B.; Ife, S.; Tar’an, B.; Vandenberg, A. CDC Tucker and CDC Leroy forage pea cultivars. Can. J. Plant Sci. 2009, 89, 661–663. [Google Scholar] [CrossRef]
- Mezlík, T.; Měřínská, S. Field Pea. Pisum sativum L.; Central Institute for Supervising and Control in Agriculture: Brno, Czech, 2010; 11p. (In Czech) [Google Scholar]
- Kreuzman, J.; Liška, M. Pea variety Terno. Czech J. Genet. Plant Breed. 2006, 42, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Mezlík, T.; Měřínská, S. Fodder Pea—Winter Type. Pisum sativum Subsp. Arvense L.; Central Institute for Supervising and Control in Agriculture: Brno, Czech, 2017; 11p. (In Czech) [Google Scholar]
- Holubec, V.; Papoušková, L.; Faberová, I.; Zedek, V.; Dotlačil, L. Framework Methodology of the National Program of Conservation and Use of Plant Genetic Resources and Agrobiodiversity; VÚRV: Prague, Czech, 2015; 386p. (In Czech) [Google Scholar]
- Germplasm Resource Information Network (GRIN) Czech Release 1.10.3. Available online: https://grinczech.vurv.cz/gringlobal/search.aspx (accessed on 4 September 2019).
- Humplík, J.F.; Lazár, L.; Fürst, T.; Husičková, A.; Hýbl, M.; Spíchal, L. Automated integrative high-throughput phenotyping of plant shoots: A case study of the cold tolerance of pea (Pisum sativum L.). Plant Methods 2015, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazár, D. Parameters of photosynthetic energy partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Systs. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Canny, J.A. Computational approach to edge detection. IEEE Trans. Pattern Anal. 1986, 8, 679–698. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Poorter, H. Avoiding bias in calculations of relative growth rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Gray, G.R.; Heath, D. A global reorganization of the metabolome in Arabidopsis during cold acclimation is revealed by metabolic fingerprinting. Physiol. Plant. 2005, 124, 236–248. [Google Scholar] [CrossRef]
- Xin, Z.; Browse, J. Cold comfort farm: The acclimation of plants to freezing temperatures. Plant Cell Environ. 2000, 23, 893–902. [Google Scholar] [CrossRef]
- de Freitas Lima, M.; Eloy, N.B.; de Siqueira, J.A.B.; Inzé, D.; Hemerly, A.S.; Ferreira, P.C.G. Molecular mechanisms of biomass increase in plants. Biotechnol. Res. Innov. 2017, 1, 14–25. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.-K.; Sunkar, R. Gene regulation during cold stress acclimation in plants. Meth. Mol. Biol. 2010, 639, 39–55. [Google Scholar]
- Dahal, K.; Kane, K.; Gadapati, W.; Webb, E.; Savitch, L.V.; Singh, J.; Sharma, P.; Sarhan, F.; Longstaffe, F.J.; Grodzinski, B.; et al. The effects of phenotypic plasticity on photosynthetic performance in winter rye, winter wheat and Brassica napus. Physiol. Plant. 2012, 144, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, P.; Iannucci, A. Winter survival of pea, faba bean and white lupin cultivars in contrasting Italian locations and sowing times, and implications for selection. J. Agric. Sci. 2007, 145, 611–622. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Dahal, K.; Bode, R.; Kurepin, L.V. Photosynthetic acclimation, vernalization, crop productivity and ‘the grand design of photosynthesis’. J. Plant Physiol. 2016, 203, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Rapacz, M.; Sasal, M.; Wójcik-Jagła, M. Direct and indirect measurements of freezing tolerance: Advantages and limitations. Acta Physiol. Plant. 2015, 37, 157. [Google Scholar] [CrossRef] [Green Version]






| Cultivar | Origin | Leaf Type | Utilization | Growth Type | Flower Color | Seed Color | Cold Resistance | Reference |
|---|---|---|---|---|---|---|---|---|
| Arkta | AGT | normal | Forage | winter | violet | brown | Central European winters | [28] |
| Enduro | AGT | afila | Field | winter | white | yellow | Mediterranean winters | [26] |
| CDC Le Roy | CDC | afila | Forage | spring | white | yellow | Canadian springs | [25] |
| Terno | AGT | afila | Field | spring | white | yellow | No | [15,27] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husičková, A.; Humplík, J.F.; Hýbl, M.; Spíchal, L.; Lazár, D. Analysis of Cold-Developed vs. Cold-Acclimated Leaves Reveals Various Strategies of Cold Acclimation of Field Pea Cultivars. Remote Sens. 2019, 11, 2964. https://doi.org/10.3390/rs11242964
Husičková A, Humplík JF, Hýbl M, Spíchal L, Lazár D. Analysis of Cold-Developed vs. Cold-Acclimated Leaves Reveals Various Strategies of Cold Acclimation of Field Pea Cultivars. Remote Sensing. 2019; 11(24):2964. https://doi.org/10.3390/rs11242964
Chicago/Turabian StyleHusičková, Alexandra, Jan F. Humplík, Miroslav Hýbl, Lukáš Spíchal, and Dušan Lazár. 2019. "Analysis of Cold-Developed vs. Cold-Acclimated Leaves Reveals Various Strategies of Cold Acclimation of Field Pea Cultivars" Remote Sensing 11, no. 24: 2964. https://doi.org/10.3390/rs11242964
APA StyleHusičková, A., Humplík, J. F., Hýbl, M., Spíchal, L., & Lazár, D. (2019). Analysis of Cold-Developed vs. Cold-Acclimated Leaves Reveals Various Strategies of Cold Acclimation of Field Pea Cultivars. Remote Sensing, 11(24), 2964. https://doi.org/10.3390/rs11242964