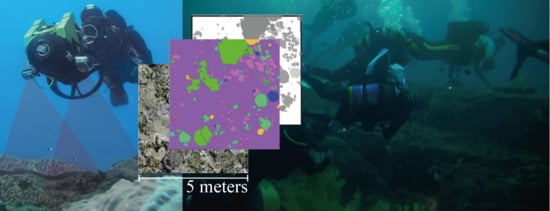
Quantifying Coral Reef Composition of Recreational Diving Sites: A Structure from Motion Approach at Seascape Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Data Processing
2.2.1. Photogrammetric Processing and Seascape Composition Analysis
2.2.2. Multivariate and Clustering Analysis
2.2.3. Estimation of Seascape Metrics
3. Results
3.1. Seascape Composition of Diving Sites
3.2. Evaluation of Clusters and Agreement with the Experts
3.3. Seascape Metrics
4. Discussion
4.1. Linking Spatial Benthic Composition to Recreational Diving Sites
4.2. Implications for Reef Management
5. Conclusions
- substantial agreement with expert opinions;
- differences in the benthic composition between diving sites in terms of: (i) organism diversity, surface, perimeter, abundance, (ii) functional groups categories (e.g., resistant, moderately resistant, fragile);
- Resistant-to-physical-impact categories (i.e., sponges and algae) were most abundant at small diving sites and highly visited by divers. These sites were characterized by big organisms and with complex shapes, low taxa diversity and density.
- Fragile-to-physical-impact categories (i.e., Acropora spp.) were most abundant at diving sites low or moderately visited by divers. These sites are characterized by complex geomorphology and moderate hydrodynamic conditions.
- The highest taxa diversity and density, and the lowest abundance of resistant-to-physical-impact FGs were recorded at large and rarely dived sites. The sites exhibited different seascape metrics (i.e., patch density, patch richness, Shannon Diversity Index, Perimeter-Area Fractal Index), with general patterns emerging in terms of responses to diving pressure and environmental conditions.
Author Contributions
Funding
Conflicts of Interest
References
- Luna, B.; Pérez, C.V.; Sánchez-Lizaso, J.L. Benthic impacts of recreational divers in a Mediterranean Marine Protected Area. ICES J. Mar. Sci. 2009, 66, 517–523. [Google Scholar] [CrossRef]
- Lyons, P.J.; Arboleda, E.; Benkwitt, C.E.; Davis, B.; Gleason, M.; Howe, C.; Mathe, J.; Middleton, J.; Sikowitz, N.; Untersteggaber, L.; et al. The effect of recreational SCUBA divers on the structural complexity and benthic assemblage of a Caribbean coral reef. Biodivers. Conserv. 2015, 24, 3491–3504. [Google Scholar] [CrossRef]
- Bravo, G.; Márquez, F.; Marzinelli, E.M.; Mendez, M.M.; Bigatti, G. Effect of recreational diving on Patagonian rocky reefs. Mar. Environ. Res. 2015, 104, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D. A brief history of recreational diving in the United States. South Pacific Underw. Med. Soc. 1999, 29. [Google Scholar]
- Buckley, R. Sustainable tourism: Research and reality. Ann. Tour. Res. 2012, 39, 528–546. [Google Scholar] [CrossRef] [Green Version]
- Dimmock, K.; Musa, G. Scuba diving tourism system: A framework for collaborative management and sustainability. Mar. Policy 2015, 54, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Roche, R.C.; Harvey, C.V.; Harvey, J.J.; Kavanagh, A.P.; McDonald, M.; Stein-Rostaing, V.R.; Turner, J.R. Recreational diving impacts on coral reefs and the adoption of environmentally responsible practices within the SCUBA diving industry. Environ. Manag. 2016, 58, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Lucrezi, S.; Saayman, M. Sustainable scuba diving tourism and resource use: Perspectives and experiences of operators in Mozambique and Italy. J. Clean. Prod. 2017, 168, 632–644. [Google Scholar] [CrossRef]
- Rogers, C.S. Responses of coral reefs and reef organisms to sedimentation. Mar. Ecol. Progress Ser. Oldendorf 1990, 62, 185–202. [Google Scholar] [CrossRef]
- Dikou, A.; Van Woesik, R. Survival under chronic stress from sediment load: Spatial patterns of hard coral communities in the southern islands of Singapore. Mar. Pollut. Bull. 2006, 52, 1340–1354. [Google Scholar] [CrossRef]
- Hawkins, J.P.; Roberts, C.M. Effects of recreational SCUBA diving on fore-reef slope communities of coral reefs. Biol. Conserv. 1992, 62, 171–178. [Google Scholar] [CrossRef]
- Chadwick-Furman, N. Effects of SCUBA diving on coral reef invertebrates in the US Virgin Islands: Implications for the management of diving. In Proceedings of the 6th International Conference on Coelenterate Biology, Noordwijkerhout, The Netherlands, 16–21 July 1995; Volume 91, pp. 1–5. [Google Scholar]
- Zakai, D.; Chadwick-Furman, N.E. Impacts of intensive recreational diving on reef corals at Eilat, northern Red Sea. Biol. Conserv. 2002, 105, 179–187. [Google Scholar] [CrossRef]
- Erftemeijer, P.L.; Riegl, B.; Hoeksema, B.W.; Todd, P.A. Environmental impacts of dredging and other sediment disturbances on corals: A review. Mar. Poll. Bull. 2012, 64, 1737–1765. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.B.; True, J.D.; Piromvaragorn, S.; Willis, B.L. Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biol. Conserv. 2014, 178, 88–96. [Google Scholar] [CrossRef]
- Wilkinson, C.R.; Green, A.; Almany, J.; Dionne, S. Monitoring Coral Reef Marine Protected Areas: A Practical Guide On How Monitoring Can Support Effective Management of MPAs; Australian Institute of Marine Science and the IUCN Marine Program: Townsville, Australia, 2003. [Google Scholar]
- Hill, J.; Wilkinson, C. Methods for ecological monitoring of coral reefs. Aust. Inst. Mar. Sci. Townsville 2004, 117, 1–116. [Google Scholar]
- Salm, R.V. Coral reefs and tourist carrying capacity; the Indian Ocean experience. Ind. Environ. 1986, 9, 11–14. [Google Scholar]
- Schleyer, M.; Celliers, L. Coral dominance at the reef–sediment interface in marginal coral communities at Sodwana Bay, South Africa. Mar. Freshw. Res. 2003, 54, 967–972. [Google Scholar] [CrossRef]
- Meesters, E.; Bos, A.; Gast, G. Effects of sedimentation and lesion position on coral tissue regeneration. In Proceedings of the Seventh International Coral Reef Symposium, Guam, Micronesia, 22–27 June 1992; Volume 2, pp. 681–688. [Google Scholar]
- Boström, C.; Pittman, S.J.; Simenstad, C.; Kneib, R.T. Seascape ecology of coastal biogenic habitats: Advances, gaps, and challenges. Mar. Ecol. Progress Ser. 2011, 427, 191–217. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Vázquez, C.; Rioja-Nieto, R.; Enriquez, C. Spatial and temporal effects of management on the reef seascape of a marine protected area in the Mexican Caribbean. Ocean Coast. Manag. 2019, 169, 50–57. [Google Scholar] [CrossRef]
- Palma, M.; Rivas Casado, M.; Pantaleo, U.; Cerrano, C. High resolution orthomosaics of African coral reefs: A tool for wide-scale benthic monitoring. Remote Sens. 2017, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Westoby, M.; Brasington, J.; Glasser, N.; Hambrey, M.; Reynolds, J. ‘Structure-from-Motion’ photogrammetry: A low-cost, effective tool for geoscience applications. Geomorphology 2012, 179, 300–314. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, R.; Figueira, W.F.; Pratchett, M.S.; Boube, T.; Adam, A.; Kobelkowsky-Vidrio, T.; Doo, S.S.; Atwood, T.B.; Byrne, M. 3D photogrammetry quantifies growth and external erosion of individual coral colonies and skeletons. Sci. Rep. 2017, 7, 16737. [Google Scholar] [CrossRef] [PubMed]
- Figueira, W.; Ferrari, R.; Weatherby, E.; Porter, A.; Hawes, S.; Byrne, M. Accuracy and precision of habitat structural complexity metrics derived from underwater photogrammetry. Remote Sens. 2015, 7, 16883–16900. [Google Scholar] [CrossRef] [Green Version]
- Storlazzi, C.D.; Dartnell, P.; Hatcher, G.A.; Gibbs, A.E. End of the chain? Rugosity and fine-scale bathymetry from existing underwater digital imagery using Structure-from-Motion (SfM) technology. Coral Reefs 2016, 35, 889–894. [Google Scholar] [CrossRef]
- Ferrari, R.; Bryson, M.; Bridge, T.; Hustache, J.; Williams, S.B.; Byrne, M.; Figueira, W. Quantifying the response of structural complexity and community composition to environmental change in marine communities. Glob. Chang. Biol. 2016, 22, 1965–1975. [Google Scholar] [CrossRef]
- Coro, G.; Palma, M.; Ellenbroek, A.; Panichi, G.; Nair, T.; Pagano, P. Reconstructing 3D virtual environments within a collaborative e-infrastructure. Concurr. Comput. Pract. Exp. 2018, 31, e5028. [Google Scholar] [CrossRef]
- Burns, J.; Delparte, D.; Kapono, L.; Belt, M.; Gates, R.; Takabayashi, M. Assessing the impact of acute disturbances on the structure and composition of a coral community using innovative 3D reconstruction tech-niques. Methods Oceanogr. 2016, 15, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Raoult, V.; David, P.A.; Dupont, S.F.; Mathewson, C.P.; O’Neill, S.J.; Powell, N.N.; Wil-liamson, J.E. GoPros™ as an underwater photogrammetry tool for citizen science. PeerJ 2016, 4, e1960. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Somerfield, P.; Clarke, K.; Warwick, R. Simpson index. In Encyclopedia of Ecology; Elsevier: Oxford, UK, 2008. [Google Scholar]
- McGarigal, K. FRAGSTATS help. Doc. FRAGSTATS 2014, 4, 1–182. [Google Scholar]
- Ramsay, P. Marine geology of the Sodwana Bay shelf, southeast Africa. Mar. Geol. 1994, 120, 225–247. [Google Scholar] [CrossRef]
- Ramsay, P. Quaternary marine geology of the Sodwana Bay continental shelf, northern KwaZulu-Natal. Oceanogr. Lit. Rev. 1996, 11, 1103. [Google Scholar]
- Jordan, I.E.; Samways, M.J. Recent changes in coral assemblages of a South African coral reef, with recommendations for long-term monitoring. Biodivers. Conserv. 2001, 10, 1027–1037. [Google Scholar] [CrossRef]
- Riegl, B. Taxonomy and Ecology of South African Reef Corals. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1993. [Google Scholar]
- Celliers, L.; Schleyer, M.H. Coral community structure and risk assessment of high-latitude reefs at Sodwana Bay, South Africa. Biodivers. Conserv. 2008, 17, 3097–3117. [Google Scholar] [CrossRef]
- Robertson, W.; Schleyer, M.; Fielding, P.; Tomalin, B.; Beckley, L.; Fennessy, S.; Van der Elst, R.; Bandeira, S.; Macia, A.; Gove, D. Inshore Marine Resources and Associated Opportunities For Development of the Coast of Southern Mozambique: Ponta do Ouro to Cabo de Santa Maria; Oceanographic Research Institute: Durban, South Africa; Volume 130, pp. 1–51, Unpublished Report.
- Ramsay, P.; Cooper, J.; Wright, C.; Mason, T. The occurrence and formation of ladderback ripples in subtidal, shallow-marine sands, Zululand, South Africa. Mari. Geol. 1989, 86, 229–235. [Google Scholar] [CrossRef]
- Riegl, B. Climate change and coral reefs: Different effects in two high-latitude areas (Arabian Gulf, South Africa). Coral Reefs 2003, 22, 433–446. [Google Scholar] [CrossRef]
- Riegl, B.; Piller, W.E. Possible refugia for reefs in times of environmental stress. Int. J. Earth Sci. 2003, 92, 520–531. [Google Scholar] [CrossRef]
- Motta, H.; Rodrigues, M.; Schleyer, M. Coral reef monitoring and management in Mozambique. In Coral Reef Degradation in the Indian Ocean: Status Report; CORDIO, SAREC Marine Science Program Department of Zoology Stockholm University: Stockholm, Sweden, 2000; pp. 43–48. [Google Scholar]
- Costa, A.; Pereira, M.A.; Motta, H.; Schleyer, M. Status of coral reefs of Mozambique: 2004. In Coral Reef Degradation in the Indian Ocean: Status Report; CORDIO, SAREC Marine Science Program Department of Zoology Stockholm University: Stockholm, Sweden, 2005; pp. 54–60. [Google Scholar]
- Roelfsema, C.; Phinn, S.; Jupiter, S.; Comley, J.; Albert, S. Mapping coral reefs at reef to reef-system scales, 10s–1000s km2, using object-based image analysis. Int. J. Remote. Sens. 2013, 34, 6367–6388. [Google Scholar] [CrossRef]
- Ferraris, D.; Palma, M.; Pantaleo, U.; Cerrano, C.; Chiantore, M. Method and Device for Tracking the Path of an Object. WO Patent App. PCT/IB2016/053,924, 5 January 2017. [Google Scholar]
- Palma, M.; Rivas Casado, M.; Pantaleo, U.; Pavoni, G.; Pica, D.; Cerrano, C. SfM-Based Method to Assess Gorgonian Forests (Paramuricea clavata (Cnidaria, Octocorallia)). Remote Sens. 2018, 10, 1154. [Google Scholar] [CrossRef] [Green Version]
- Peraira, M.A.; Schleyer, M.H. A diver and diving survey in southern Mozambique. In Coral Reef Degrad. Indian Ocean: Status Report; CORDIO, SAREC Marine Science Program Department of Zoology Stockholm University: Stockholm, Sweden, 2005; pp. 184–192. [Google Scholar]
- Edinger, E.N.; Risk, M.J. Reef classification by coral morphology predicts coral reef conservation value. Biol. Conserv. 2000, 92, 1–13. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulff, J. Resistance vs recovery: Morphological strategies of coral reef sponges. Funct. Ecol. 2006, 20, 699–708. [Google Scholar] [CrossRef]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative states on coral reefs: Beyond coral–macroalgal phase shifts. Mar. Ecol. Progress Ser. 2009, 376, 295–306. [Google Scholar] [CrossRef]
- Riegl, B.; Schleyer, M.H.; Cook, P.; Branch, G. Structure of Africa’s southernmost coral communities. Bull. Mar. Sci. 1995, 56, 676–691. [Google Scholar]
- Schleyer, M.H.; Tomalin, B.J. Damage on South African coral reefs and an assessment of their sustainable diving capacity using a fisheries approach. Bull. Mar. Sci. 2000, 67, 1025–1042. [Google Scholar]
- Rouphael, T.; Inglis, G. The effects of qualified recreational SCUBA divers on coral reefs. Tech. Rep. CRC Reef Res. Cent. 1995, 4, 39. [Google Scholar]
- Floros, C.; Schleyer, M. The functional importance of Acropora austera as nursery areas for juvenile reef fish on South African coral reefs. Coral Reefs 2017, 36, 139–149. [Google Scholar] [CrossRef]
- Hughes, T.; Connell, J. Multiple stressors on coral reefs: A long-term perspective. Limnol. Oceanogr. 1999, 44, 932–940. [Google Scholar] [CrossRef]
- Jolliffe, I. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/0470013192.bsa501 (accessed on 16 October 2019).
- Pelleg, D.; Moore, A.W. X-means: Extending k-means with efficient estimation of the number of clusters. Icml 2000, 1, 727–734. [Google Scholar]
- Coro, G.; Candela, L.; Pagano, P.; Italiano, A.; Liccardo, L. Parallelizing the execution of native data mining algorithms for computational biology. Concurr. Comput. Pract. Exp. 2015, 27, 4630–4644. [Google Scholar] [CrossRef]
- Coro, G.; Panichi, G.; Scarponi, P.; Pagano, P. Cloud computing in a distributed e-infrastructure using the web processing service standard. Concurr. Comput. Pract. Exp. 2017, 29, e4219. [Google Scholar] [CrossRef]
- Magliozzi, C.; Coro, G.; Grabowski, R.; Packman, A.I.; Krause, S. A multiscale statistical method to identify potential areas of hyporheic exchange for river restoration planning. Environ. Modell. Softw. 2019, 111, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Measur. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Coro, G.; Webb, T.J.; Appeltans, W.; Bailly, N.; Cattrijsse, A.; Pagano, P. Classifying degrees of species commonness: North Sea fish as a case study. Ecol. Modell. 2015, 312, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Garrabou, J.; Riera, J.; Zabala, M. Landscape pattern indices applied to Mediterranean subtidal rocky benthic communities. Landsc. Ecol. 1998, 13, 225–247. [Google Scholar] [CrossRef]
- Teixido, N.; Garrabou, J.; Arntz, W. Spatial pattern quantification of Antarctic benthic communities using landscape indices. Mar. Ecol. Progress Ser. 2002, 242, 1–14. [Google Scholar] [CrossRef]
- Kendall, M.S.; Miller, T. The influence of thematic and spatial resolution on maps of a coral reef ecosystem. Mar. Geodesy 2008, 31, 75–102. [Google Scholar] [CrossRef]
- Wedding, L.M.; Lepczyk, C.A.; Pittman, S.J.; Friedlander, A.M.; Jorgensen, S. Quantifying seascape structure: Extending terrestrial spatial pattern metrics to the marine realm. Mar. Ecol. Progress Ser. 2011, 427, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Chambers, J.M.; Hastie, T.J. Statistical Models in S; Wadsworth & Brooks/Cole Advanced Books & Software: Pacific Grove, CA, USA, 1992; Volume 251. [Google Scholar]
- Zuur, A.; Ieno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer Science+: New York, NY, USA, 2009. [Google Scholar]
- Stats, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Fox, J.; Weisberg, S.; Friendly, M.; Hong, J.; Andersen, R.; Firth, D.; Taylor, S.; Team, R.C.; Fox, M.J. Package ‘Effects’; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Gladfelter, E.H.; Monahan, R.K.; Gladfelter, W.B. Growth rates of five reef-building corals in the northeastern Caribbean. Bull. Mar. Sci. 1978, 28, 728–734. [Google Scholar]
- Dornelas, M.; Madin, J.S.; Baird, A.H.; Connolly, S.R. Allometric growth in reef-building corals. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170053. [Google Scholar] [CrossRef] [Green Version]
- Schumann, E.; Orren, M. The Physico-Chemical Characteristics of the South-West Indian Ocean in Relation to Maputaland; Rhodes University and The Wildlife Society of Southern Africa: Grahamstown-Durban, South Africa, 1980; pp. 8–11. [Google Scholar]
- Lirman, D. Fragmentation in the branching coral Acropora palmata (Lamarck): Growth, survivorship, and reproduction of colonies and fragments. J. Exp. Mar. Biol. Ecol. 2000, 251, 41–57. [Google Scholar] [CrossRef]
- Schleyer, M.H.; Kruger, A.; Celliers, L. Long-term community changes on a high-latitude coral reef in the Greater St Lucia Wetland Park, South Africa. Mar. Poll. Bull. 2008, 56, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Lucrezi, S.; Saayman, M.; van der Merwe, P. Managing diving impacts on reef ecosystems: Analysis of putative influences of motivations, marine life preferences and experience on divers’ environmental perceptions. Ocean Coast. Manag. 2013, 76, 52–63. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.V.; Harvey, J.J.; Miller, A.; Johnson, V.; Phongsuwan, N. The Green Fins approach for monitoring and promoting environmentally sustainable scuba diving operations in South East Asia. Ocean Coast. Manag. 2013, 78, 35–44. [Google Scholar] [CrossRef]
- Lucrezi, S.; Milanese, M.; Palma, M.; Cerrano, C. Stirring the strategic direction of scuba diving marine Citizen Science: A survey of active and potential participants. PLoS ONE 2018, 13, e0202484. [Google Scholar] [CrossRef]
- Loerzel, J.L.; Goedeke, T.L.; Dillard, M.K.; Brown, G. SCUBA divers above the waterline: Using participatory mapping of coral reef conditions to inform reef management. Mar. Policy 2017, 76, 79–89. [Google Scholar] [CrossRef]
- Cerrano, C.; Milanese, M.; Ponti, M. Diving for science-science for diving: Volunteer scuba divers support science and conservation in the Mediterranean Sea. Aquatic Conserv. Mar. Freshw. Ecosyst. 2017, 27, 303–323. [Google Scholar] [CrossRef]
- Magris, R.A.; Pressey, R.L.; Weeks, R.; Ban, N.C. Integrating connectivity and climate change into marine conservation planning. Biol. Conserv. 2014, 170, 207–221. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827. [Google Scholar] [CrossRef]









| Index | Description | Number of Dives/yr | Site Area | Site Depth | |||||
|---|---|---|---|---|---|---|---|---|---|
| High (>3k Dives/yr) | Medium (>1k Dives / yr; <3k Dives/yr) | Small (<1k Dives/yr) | Low (<850 m) | Large (>850 m) | Shallow (>−18 m) | Deep (<−18 m) | |||
| Geometry | Patch surface | The patch surface is the area occupied by an organism | medium to large | small– large | small– large | large | small– large | Large | small– large |
| Patch perimeter | The patch perimeter is the length of the planar borders of an organisms | complex | simple– complex | simple– complex | simple | simple– complex | complex | simple– complex | |
| Aggregation | PD * (Patch density) | Indicates the complexity of the seascape with no reference to the diversity of classes and the size of the patches. PD is the number of patches counted within the landscape. | low | medium- high | high | low | high | low- medium | high |
| Diversity | SIDI * (Simpson Diversity Index) | Indicates the probability that two entities, (i.e., pixels) taken at random from the same seascape, belong to different patch types. Large values indicate high probability that two pixels are from different patch types [33]. | low | medium- high | high | low | high | low- medium | high |
| PR * (Patch Richness) | Indicates the number of patch classes present within the seascape. | low | medium- high | high | low | high | low- medium | high | |
| SHDI * (Shannon’s Diversity Index) | Indicates the number of different patch types within the seascape and their evenness. Large SHDI values indicate high evenness among patch types within the seascape [32]. | low | medium- high | high | low | high | low- medium | high | |
| Shape | PAFRAC * (Perimeter- area fractal dimension) | Indicates the complexity of the organisms across an area. e.g., if small and large patches have similar and simple geometric shapes, the index will be small, indicating that as the patch area increases, the patch perimeter increases too but by small increment [34]. | low | medium- high | high | low | high | low | high |
| FRAC_MN * (Mean patch fractal dimension) | Balances the PAFRAC results when the patch frequency is <20 [34] by calculating an average fractal dimension of each patch. | high to medium | medium- low | medium- low | high– medium | medium– low | high– medium | medium– low | |
| Year | Blacks | Doodles | Maverick | Paradise Ledge | Steps | Techobanine | Texas |
|---|---|---|---|---|---|---|---|
| 2001 * | 8419 | 1955 | 2542 | 2286 | |||
| 2002 * | 12,282 | 2852 | 3708 | 4210 | |||
| 2011 ** | 761 | 5644 | 187 | 3213 | 407 | ||
| 2012 ** | 1257 | 9737 | 34 | 366 | 3957 | 305 | |
| 2013 ** | 869 | 8474 | 388 | 4369 | 127 | ||
| 2014 ** | 668 | 7117 | 14 | 176 | 3477 | 222 | |
| 2015 ** | 1174 | 6105 | 563 | 3042 | 35 | 305 | |
| 2016 ** | 1184 | 7168 | 14 | 512 | 2714 | 318 |
| PPMR Bays | Dive Sites | Long., Lat. (WGS84) | Distance from Launch (km) | Average Depth (m) | Depth Range (m) | Area (m2) | Average Dives Year−1 (2001–2002) | Average Dives Year−1 (2011–2016) |
|---|---|---|---|---|---|---|---|---|
| Ponta do Ouro | Doodles | W 32.896103, N −26.830669 | 1.7 | 17 | 16–18 | 850 | 10350 | 7374 |
| Blacks | W 32.897408, N −26.824883 | 2.5 | 19 | 18–20 | 425 | - | 985 | |
| Steps | W 32.894828, N −26.813275 | 3.6 | 16 | 15–17 | 950 | 3125 | 3462 | |
| Ponta Malongane | Maverick | W 32.904875, N −26.775608 | 7.9 | 25 | 23–26 | 2550 | - | 20 |
| Paradise Ledge | W 32.903339, N −26.780397 | 7.1 | 22 | 21–23 | 2250 | 2403 | 365 | |
| Texas * | W 32.902742, N −26.763153 | 9.5 | 15 | 14–16 | 2150 | 3248 | 280 | |
| Ponta Techobanine | Techobanine * | W 32.903397, N −26.677103 | 19.6 | 12 | 8–15 | 3500 | - | 35 |
| Resistant-to-Physical-Impact Categories | Functional Groups (FGs) | Classes | Code |
|---|---|---|---|
| Fragile | Acropora spp. | Acropora branched | ACB |
| Acropora | ACC | ||
| Acropora digitate | ACD | ||
| Acropora stout branched | ACS | ||
| Non-Acropora branched coral | Non-Acropora branched coral | CB | |
| Free-living fungiid | Free-living fungiid | CMR | |
| Tabular coral | Tabular coral | CTA | |
| Moderately fragile | Encrusting coral | Encrusting coral | CE |
| Folious coral | Folious coral | CF | |
| Massive coral | Massive coral | CM | |
| Soft corals | Soft crested coral | SCR | |
| Soft digitate coral | SCD | ||
| Soft mushroom coral | SCF | ||
| Soft plane coral | SCP | ||
| Soft radiate coral | SCR | ||
| Resistant | Other invertebrate | Other invertebrate | OI |
| Sponges | Sponge | SP | |
| Sponge encrusting | SPE | ||
| Sponge massive | SPM | ||
| Algal turf | Algal turf | TA |
| Classes | Doodles | Blacks | Steps | Paradise Ledge | Maverick | Texas | Techobanine | |
|---|---|---|---|---|---|---|---|---|
| Average organisms’ density individuals/m2 | ACB | 0.00 | 0.00 | 0.00 | 0.03 | 0.11 | 0.02 | 0.09 |
| ACC | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.57 | |
| ACD | 0.00 | 0.00 | 0.00 | 0.47 | 0.07 | 0.00 | 0.00 | |
| ACS | 0.00 | 0.00 | 0.03 | 0.19 | 0.16 | 0.15 | 0.20 | |
| CB | 0.00 | 0.00 | 0.31 | 0.68 | 0.16 | 0.73 | 0.56 | |
| CE | 0.01 | 0.00 | 0.03 | 0.04 | 0.09 | 0.02 | 0.01 | |
| CF | 0.01 | 0.07 | 0.08 | 0.07 | 0.25 | 0.02 | 0.12 | |
| CM | 0.00 | 0.00 | 0.01 | 0.09 | 0.11 | 0.04 | 0.24 | |
| CMR | 0.00 | 0.00 | 0.00 | 0.17 | 0.07 | 0.00 | 0.00 | |
| CTU | 0.04 | 0.03 | 0.21 | 0.15 | 0.41 | 0.27 | 0.27 | |
| OI | 0.00 | 0.00 | 0.58 | 0.11 | 0.00 | 0.00 | 0.00 | |
| SCC | 0.07 | 0.00 | 0.29 | 1.01 | 0.33 | 1.45 | 0.43 | |
| SCD | 0.11 | 0.00 | 0.49 | 1.80 | 1.59 | 1.66 | 0.55 | |
| SCF | 0.01 | 0.00 | 0.01 | 0.17 | 0.31 | 0.63 | 0.33 | |
| SCP | 0.01 | 0.00 | 0.01 | 0.05 | 0.31 | 0.00 | 0.00 | |
| SCR | 0.03 | 0.00 | 0.08 | 0.11 | 0.29 | 0.73 | 0.11 | |
| SP | 0.00 | 0.19 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | |
| SPE | 0.03 | 0.08 | 0.07 | 0.00 | 0.00 | 0.59 | 0.05 | |
| SPM | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.03 | 0.00 | |
| TA | 0.16 | 0.11 | 0.16 | 0.00 | 0.13 | 0.13 | 0.03 | |
| Total | 0.48 | 0.48 | 2.37 | 4.85 | 4.40 | 6.47 | 3.56 |
| Clusters | Label | Description |
|---|---|---|
| Cluster 1 | Very impacted | Distribution of organisms patches with large average surface and perimeter in relation to the total abundance. High abundance of algal patches with large perimeter. Abundant average sponge patches with large maximal perimeter length and high variability among patches. No Acropora corals, branched corals and massive corals patches. Folios coral patches with large perimeter. Low abundance of tabular coral patches. Rare soft crested coral patches with small maximal surface and perimeter. Very low abundance of soft digitate coral patches. Rare and soft mushroom coral with small average areas and medium average perimeter. |
| Cluster 2 | Moderately impacted | Distribution of organism patches with small average surface and perimeter in relation to the total abundance. Medium abundance of algal patches with medium perimeter. Abundant average sponge patches with medium maximal perimeter and low variability among patches. Medium abundance of Acropora patches with medium average perimeter. Large average branched coral patches surface with large perimeter. Folios coral patches with medium perimeter. Massive coral with medium patch areas and perimeters. Medium abundance of tabular coral patches. Medium soft crested coral patches maximal surface with large maximal perimeter. Medium abundance of soft digitate coral patches. Soft mushroom coral with large average areas and large average perimeter. |
| Cluster 3 | Low impacted | Distribution of organism patches with very small average surface and perimeter in relation to the total abundance. Low abundance of algal patches with small perimeter. Rare sponges with very small perimeter length. High abundance of Acropora coral patches with medium average perimeter. Small average branched coral patches surface with small perimeter. Folios coral patches with small perimeter. Massive coral with medium patch areas and perimeters. High abundance of tabular coral patches. Large soft crested coral patches maximal surface with medium maximal perimeter. High abundance of soft digitate coral patches. Soft mushroom coral with medium average areas and medium average perimeter. |
| Expert 2 | Clustering | |
|---|---|---|
| Kappa values | ||
| Expert 1 | 0.36 | 0.78 |
| Expert 2 | 0.57 | |
| Kappa interpretation Fleiss/Landis–Koch | ||
| Expert 1 | Marginal | Excellent/substantial |
| Expert 2 | Good/Moderate | |
| Absolute percentage of agreement | ||
| Expert 1 | 57% | 86% |
| Expert 2 | 71% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, M.; Magliozzi, C.; Rivas Casado, M.; Pantaleo, U.; Fernandes, J.; Coro, G.; Cerrano, C.; Leinster, P. Quantifying Coral Reef Composition of Recreational Diving Sites: A Structure from Motion Approach at Seascape Scale. Remote Sens. 2019, 11, 3027. https://doi.org/10.3390/rs11243027
Palma M, Magliozzi C, Rivas Casado M, Pantaleo U, Fernandes J, Coro G, Cerrano C, Leinster P. Quantifying Coral Reef Composition of Recreational Diving Sites: A Structure from Motion Approach at Seascape Scale. Remote Sensing. 2019; 11(24):3027. https://doi.org/10.3390/rs11243027
Chicago/Turabian StylePalma, Marco, Chiara Magliozzi, Monica Rivas Casado, Ubaldo Pantaleo, João Fernandes, Gianpaolo Coro, Carlo Cerrano, and Paul Leinster. 2019. "Quantifying Coral Reef Composition of Recreational Diving Sites: A Structure from Motion Approach at Seascape Scale" Remote Sensing 11, no. 24: 3027. https://doi.org/10.3390/rs11243027
APA StylePalma, M., Magliozzi, C., Rivas Casado, M., Pantaleo, U., Fernandes, J., Coro, G., Cerrano, C., & Leinster, P. (2019). Quantifying Coral Reef Composition of Recreational Diving Sites: A Structure from Motion Approach at Seascape Scale. Remote Sensing, 11(24), 3027. https://doi.org/10.3390/rs11243027