Breeding Colony Dynamics of Southern Elephant Seals at Patelnia Point, King George Island, Antarctica
Abstract
:1. Introduction
2. Materials and Methods
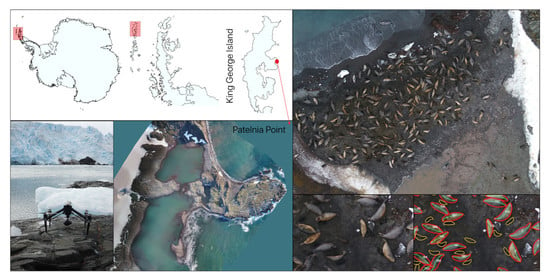
2.1. Study Area
2.2. Unmanned Aerial Vehicle (UAV) Flight Parameters
2.3. Time-Lapse Photography and Ground Counts
2.4. Image Processing and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- This paper confirmed that counting southern elephant seals using UAVs is more accurate than counting by humans, and it is therefore strongly recommended that drones be used to monitor breeding colonies of southern elephant seals.
- The body surface area of southern elephant seals can be used to assess the development of a particular zone, as it is associated with body mass lost due to parturition and latency in females.
- Harems are highly dynamic and unstable groups (in the sense of spatial changes and individuals movement), so the situation considered here is temporary. To better understand reproductive dynamics, all interactions between existing harems and their transformations should also be taken into account. For this reason, it is better to consider the belonging of an individual to a certain hermetic area (zone) than to a specific harem. Zones provide an opportunity to determine the phase of the phenological cycle.
- Based on body length measurements of female southern elephant seals, we suggest assessing the age of such females using the well-known power equation that links body length and age. Our calculations showed that the mean age of females involved in breeding at Patelnia was 7.58 years with a standard deviation equal to 3.04 years.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Factor | Investigated Characteristics | ANOVA | Levene’s Test | Post-Hoc Test | ||
|---|---|---|---|---|---|---|
| df | F | p | ||||
| Zone I | Body Length | 8 | 0.941 | 0.481 | 0.178 | none |
| Body Surf. | 8 | 9.778 | 0.000 | 0.083 | Tukey’s | |
| Zone II | Body Length | 8 | 1.725 | 0.092 | 0.340 | none |
| Body Surf. | 8 | 3.403 | 0.001 | 0.268 | Tukey’s | |
| Zone III | Body Length | 6 | 1.735 | 0.114 | 0.935 | none |
| Body Surf. | 6 | 7.198 | 0.000 | 0.991 | Tukey’s | |
| Zone IV | Body Length | 6 | 2.339 | 0.031 | 0.093 | Tukey’s |
| Body Surf. | 6 | 5.673 | 0.000 | 0.010 | Tamhane’s | |
| October 13 | Body Length | 1 | 7.117 | 0.008 | 0.059 | LSD |
| Body Surf. | 1 | 5.258 | 0.023 | 0.628 | LSD | |
| October 15 | Body Length | 3 | 3.007 | 0.031 | 0.317 | LSD |
| Body Surf. | 3 | 1.543 | 0.203 | 0.375 | none | |
| October 19 | Body Length | 3 | 3.053 | 0.028 | 0.404 | LSD |
| Body Surf. | 3 | 0.730 | 0.534 | 0.493 | none | |
| October 24 | Body Length | 3 | 1.887 | 0.131 | 0.841 | none |
| Body Surf. | 3 | 0.488 | 0.691 | 0.332 | none | |
| October 27 | Body Length | 3 | 5.981 | 0.001 | 0.646 | LSD |
| Body Surf. | 3 | 3.767 | 0.011 | 0.291 | LSD | |
| November 1 | Body Length | 3 | 0.391 | 0.760 | 0.853 | none |
| Body Surf. | 3 | 0.790 | 0.500 | 0.670 | none | |
| November 4 | Body Length | 3 | 2.978 | 0.032 | 0.297 | LSD |
| Body Surf. | 3 | 1.141 | 0.333 | 0.229 | none | |
| November 7 | Body Length | 3 | 1.142 | 0.333 | 0.591 | none |
| Body Surf. | 3 | 0.154 | 0.927 | 0.043 | none | |
| November 16 | Body Length | 3 | 5.131 | 0.004 | 0.007 | Tamhane’s |
| Body Surf. | 3 | 3.326 | 0.027 | 0.308 | LSD | |
| November 26 | Body Length | 3 | 2.335 | 0.099 | 0.266 | none |
| Body Surf. | 3 | 1.943 | 0.150 | 0.863 | none | |
References
- Rogers, A.D.; Frinault, B.A.V.; Barnes, D.K.A.; Bindoff, N.L.; Downie, R.; Ducklow, H.W.; Friedlaender, A.S.; Hart, T.; Hill, S.L.; Hofmann, E.E.; et al. Antarctic Futures: An Assessment of Climate-Driven Changes in Ecosystem Structure, Function, and Service Provisioning in the Southern Ocean. Annu. Rev. Mar. Sci. 2020, 12, 87–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slade, R.W.; Moritz, C.; Hoelzel, A.R.; Burton, H.R. Molecular population genetics of the southern elephant seal Mirounga leonina. Genetics 1998, 149, 1945–1957. [Google Scholar] [PubMed]
- Corrigan, L.J.; Fabiani, A.; Chauke, L.F.; McMahon, C.R.; De Bruyn, M.; Bester, M.N.; Bastos, A.D.S.; Campagna, C.; Muelbert, M.M.; Hoelzel, A.R. Population differentiation in the context of Holocene climate change for a migratory marine species, the southern elephant seal. J. Evol. Biol. 2016, 29, 1667–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindell, M.A.; McMahon, C.R.; Bester, M.N.; Boehme, L.; Costa, D.; Fedak, M.A.; Guinet, C.; Herraiz-Borreguero, L.; Harcourt, R.; Hückstädt, L.A.; et al. Circumpolar habitat use in the southern elephant seal: Implications for foraging success and population trajectories. Ecosphere 2016, 7, 01213. [Google Scholar] [CrossRef] [Green Version]
- McMahon, C.R.; Bester, M.N.; Burton, H.R.; Hindell, M.A.; Bradshaw, C.J. Population status, trends and a re-examination of the hypotheses explaining the recent declines of the southern elephant seal Mirounga leonina. Mammal Rev. 2005, 35, 82–100. [Google Scholar] [CrossRef]
- Aguayo, A.L. Census of pinnipedia in the South Shetland Islands. In Antarctic Ecology; Holdgate, M.W., Ed.; Academic Press: London, UK, 1970; pp. 395–397. [Google Scholar]
- Gil-Delgado, J.A.; Villaescusa, J.A.; Diazmacip, M.E.; Velázquez, D.; Rico, E.; Toro, M.; Quesada, A.; Camacho, A. Minimum population size estimates demonstrate an increase in southern elephant seals (Mirounga leonina) on Livingston Island, maritime Antarctica. Polar Biol. 2012, 36, 607–610. [Google Scholar] [CrossRef]
- Carlini, A.R.; Poljak, S.; Casaux, R.; Daneri, G.A.; Gasco, M. Southern elephant seals breeding at Nelson Island, South Shetland Islands. Pol. Polar Res. 2003, 24, 143–147. [Google Scholar]
- Salwicka, K.; Rakusa-Suszczewski, S. Long-term monitoring of Antarctic pinnipeds in Admiralty Bay (South Shetlands, Antarctica). Acta Thériol. 2002, 47, 443–457. [Google Scholar] [CrossRef]
- Vergani, D.F.; Stanganelli, Z.B. Fluctuations in Breeding Populations of Elephant Seals Mirounga leonina at Stranger Point, King George Island 1980–1988. In Antarctic Ecosystems; Kerry, K.R., Hempel, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Carlini, A.R.; Polijak, S.; Daneri, G.A.; Márquez, M.E.I.; Plötz, J. Dynamics of male dominate of southern elephant seals (Mirounga leonine) during breeding season at King George Island. Pol. Polar Res. 2002, 23, 153–159. [Google Scholar]
- Carlini, A.R.; Márquez, M.E.I.; Soave, G.; Vergani, D.F.; De Ferrer, P.A.R. Southern elephant seal, Mirounga leonina: Composition of milk during lactation. Polar Biol. 1994, 14, 37–42. [Google Scholar] [CrossRef]
- Carlini, A.R.; Daneri, G.A.; Márquez, M.E.I.; Soave, G.E.; Poljak, S. Mass transfer from mothers to pups and mass recovery by mothers during the post-breeding foraging period in southern elephant seals (Mirounga leonina) at King George Island. Polar Biol. 1997, 18, 305–310. [Google Scholar] [CrossRef]
- Daneri, G.; Carlini, A. Fish prey of southern elephant seals, Mirounga leonina, at King George Island. Polar Biol. 2002, 25, 739–743. [Google Scholar] [CrossRef]
- Daneri, G.A.; Carlini, A.R.; Marschoff, E.R.; Harrington, A.; Negrete, J.; Mennucci, J.A.; Márquez, M.E.I. The feeding habits of the Southern elephant seal, Mirounga leonina, at Isla 25 de Mayo/King George Island, South Shetland Islands. Polar Biol. 2014, 38, 665–676. [Google Scholar] [CrossRef]
- Gerber, L.R. Including behavioral data in demographic models improves estimates of population viability. Front. Ecol. Environ. 2006, 4, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Skinner, J.D.; Van Aarde, R.J. Observations on the Trend of the Breeding Population of Southern Elephant Seals, Mirounga leonina, at Marion Island. J. Appl. Ecol. 1983, 20, 707. [Google Scholar] [CrossRef]
- Galimberti, F.; Boitani, L. Demography and breeding biology of a small localized population of Southern elephant seals (Mirounga leonina). Mar. Mammal Sci. 1999, 15, 159–178. [Google Scholar] [CrossRef]
- Van Aarde, R.J. Harem structure of the southern elephant seal Mirounga leonina at Kerguelen Island. Revue d’Ecologie 1980, 34, 41–44. [Google Scholar]
- McMahon, C.R.; Bradshaw, C.J. Harem choice and breeding experience of female southern elephant seals influence offspring survival. Behav. Ecol. Sociobiol. 2004, 55, 349–362. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Pettorelli, N.; Nagendra, H.; Rocchini, D.; Rowcliffe, M.; Williams, R.; Ahumada, J.; De Angelo, C.; Atzberger, C.; Boyd, D.S.; Buchanan, G.; et al. Remote Sensing in Ecology and Conservation: Three years on. Remote. Sens. Ecol. Conserv. 2017, 3, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, C.; Lejeune, P.; Lisein, J.; Sawadogo, P.; Bouché, P. Unmanned Aerial Survey of Elephants. PLoS ONE 2013, 8, e54700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmarz, A.; Rodzewicz, M.; Dąbski, M.; Karsznia, I.; Korczak-Abshire, M.; Chwedorzewska, K.J. Application of UAV BLOS remote sensing data for multi-faceted analysis of Antarctic ecosystem. Remote Sens. Environ. 2018, 217, 375–388. [Google Scholar] [CrossRef]
- Korczak-Abshire, M.; Zmarz, A.; Rodzewicz, M.; Kycko, M.; Karsznia, I.; Chwedorzewska, K.J. Study of fauna population changes on Penquin Island and Turret Point oasis (King George Island, Antarctica) using an unmanned aerial vehicle. Polar Biol. 2019, 42, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, O.; Braun, C.; Esefeld, J.; Knetsch, S.; Maercker, J.; Pfeifer, C.; Rummler, M.C. Detecting Antarctic seals and flying seabirds by UAV. ISPRS Ann. Photogramm. Remote. Sens. Spat. Inf. Sci. 2019, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, C.; Barbosa, A.; Mustafa, O.; Peter, H.U.; Brenning, A.; Rummler, M.C. Using fixed wing UAV for Detecting and mapping the distribution and abundance of penquins on the South Shetlands Islands. Antarctica. Drones 2019, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Goebel, M.E.; Perryman, W.L.; Hinke, J.T.; Krause, D.J.; Hann, N.A.; Gardner, S.; Leroi, D.J. A small unmanned aerial system for estimating abundance and size of Antarctic predators. Polar Biol. 2015, 38, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Hinke, J.T.; Barbosa, A.; Emmerson, L.; Hart, T.; Juáres, M.A.; Korczak-Abshire, M.; Milinevsky, G.; Santos, M.; Trathan, P.N.; Watters, G.M.; et al. Estimating nest-level phenology and reproductive success of colonial seabirds using time-lapse cameras. Methods Ecol. Evol. 2018, 9, 1853–1863. [Google Scholar] [CrossRef] [Green Version]
- McMahon, C.R.; Howe, H.; Hoff, J.V.D.; Alderman, R.; Brolsma, H.; Hindell, M.A. Satellites, the All-Seeing Eyes in the Sky: Counting Elephant Seals from Space. PLoS ONE 2014, 9, e92613. [Google Scholar] [CrossRef] [Green Version]
- LaRue, M.; Ainley, D.G.; Pennycook, J.; Stamatiou, K.; Salas, L.; Nur, N.; Stammerjohn, S.; Barrington, L. Engaging ‘the crowd’ in remote sensing to learn about habitat affinity of the Weddell seal in Antarctica. Remote. Sens. Ecol. Conserv. 2019, 6, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Adame, K.; Pardo, M.A.; Salvadeo, C.; Beier, E.; Elorriaga-Verplancken, F.R. Detectability and categorization of California sea lions using an unmanned aerial vehicle. Mar. Mammal Sci. 2017, 33, 913–925. [Google Scholar] [CrossRef]
- Lowry, M.S. Count of California sea lion (Zalophus californianus) pups from aerial color photographs and from the ground: A comparison of two methods. Mar. Mammal Sci. 1999, 15, 143–158. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Mott, R.; Baylis, S.; Pham, T.; Wotherspoon, S.; Kilpatrick, A.D.; Segaran, R.R.; Reid, I.; Terauds, A.; Koh, L.P. Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef] [Green Version]
- Blindow, N.; Suckro, S.; Rückamp, M.; Braun, M.; Schindler, M.; Breuer, B.; Saurer, H.; Simões, J.C.; Lange, M. Geometry and status of the King George Island ice cap (South Shetland Islands, Antarctica). Ann. Glaciol. 2010, 51, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Krzemiński, W. Southern elephant seal (Mirounga leonina L.) of Admiralty Bay (King George Islands). Its numbers and activity during the moulting season in summer 1978/1979. Pol. Polar Res. 1981, 2, 143–152. [Google Scholar]
- Plenzler, J.; Budzik, T.; Puczko, D.; Bialik, R.J. Climatic conditions at Arctowski Station (King George Island, West Antarctica) in 2013–2017 against the background of regional changes. Pol. Polar Res. 2019, 40, 1–27. [Google Scholar]
- Mustafa, O.; Barbosa, A.; Krause, D.J.; Peter, H.-U.; Vieira, G.; Rümmler, M.-C. State of knowledge: Antarctic wildlife response to unmanned aerial systems. Polar Biol. 2018, 41, 2387–2398. [Google Scholar] [CrossRef]
- Harris, C.M.; Herata, H.; Hertel, F. Environmental guidelines for operation of Remotely Piloted Aircraft Systems (RPAS): Experience from Antarctica. Biol. Conserv. 2019, 236, 521–531. [Google Scholar] [CrossRef]
- Krause, D.J.; Hinke, J.T.; Perryman, W.L.; Goebel, M.E.; Leroi, D.J. An accurate and adaptable photogrammetric approach for estimating the mass and body condition of pinnipeds using an unmanned aerial system. PLoS ONE 2017, 12, e0187465. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.M.; Burton, H.R.; Lea, M.-A.; Hindell, M.A. Growth of female southern elephant seals Mirounga leonina at Macquarie Island. Polar Biol. 2005, 28, 395–401. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Baylis, S.; Mott, R.; Herrod, A.; Clarke, R.H. Precision wildlife monitoring using unmanned aerial vehicles. Sci. Rep. 2016, 6, 22574. [Google Scholar] [CrossRef] [Green Version]
- Sorrell, K.; Clarke, R.H.; Holmberg, R.; McIntosh, R.R. Remotely piloted aircraft improve precision of capture–mark–resight population estimates of Australian fur seals. Ecosphere 2019, 10, 02812. [Google Scholar] [CrossRef] [Green Version]
- Muller-Schwarze, D.; Waltz, E.C.; Trivelpiece, W.; Volkman, N.J. Breeding status of southern elephant seals at King George Island. Antarct. J. U. S. 1978, 13, 157–158. [Google Scholar]
- Galimberti, F.; Sanvito, S. Modeling female haul-out in southern elephant seals (Mirounga leonina). Aquat. Mamm. 2001, 27, 92–104. [Google Scholar]
- Pudełko, R.; Angiel, P.J.; Potocki, M.; Jędrejek, A.; Kozak, M. Fluctuation of Glacial Retreat Rates in the Eastern Part of Warszawa Icefield, King George Island, Antarctica, 1979–2018. Remote. Sens. 2018, 10, 892. [Google Scholar] [CrossRef] [Green Version]
- Da Rosa, K.K.; Perondi, C.; Veettil, B.K.; Auger, J.D.; Simões, J.C. Contrasting responses of land-terminating glaciers to recent climate variations in King George Island, Antarctica. Antarct. Sci. 2020, 1–10. [Google Scholar] [CrossRef]











| Mission No. | Mission Date (2019) | Area Coverage | Number of Images Taken (Calibrated) | Flight Altitude | Image Overlap | Ground Sampling Distance (GSD)—Pixel Resolution |
|---|---|---|---|---|---|---|
| 1 | October 13 | 0.104 km2 | 910 (729) | 50 m | 85–75% | 1.04 cm |
| 2 | October 15 | 0.237 km2 | 1375 (1231) | 75 m | 85–75% | 1.57 cm |
| 3 | October 19 | 0.200 km2 | 1218 (984) | 65 m | 85–75% | 1.36 cm |
| 4 | October 24 * | 0.203 km2 | 890 (741) | 65 m | 85–75% | 1.39 cm |
| 5 | October 27 | 0.253 km2 | 1217 (1074) | 65 m | 85–75% | 1.50 cm |
| 6 | November 1 | 0.181 km2 | 1211 (865) | 65 m | 85–75% | 1.34 cm |
| 7 | November 4 | 0.203 km2 | 1217 (963) | 65 m | 85–75% | 1.32 cm |
| 8 | November 7 | 0.192 km2 | 1261 (890) | 65 m | 85–75% | 1.39 cm |
| 9 | November 16 | 0.179 km2 | 1425 (863) | 65 m | 85–70% | 1.42 cm |
| 10 | November 19 | 0.224 km2 | 1410 (1009) | 65 m | 85–75% | 1.39 cm |
| 11 | November 26 | 0.338 km2 | 1399 (1184) | 65 m | 85–70% | 1.44 cm |
| 12 | December 6 | 0.348 km2 | 1702 (1181) | 65 m | 80–75% | 1.37 cm |
| Data | Zone I | Zone II | Zone III | Zone IV | Patelnia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | M | F | P | M | F | P | M | F | P | M | F | P | M | |
| October 1 | 53 | 11 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 53 | 11 | 6 |
| October 11 | 140 | 85 | 4 | 6 | 1 | 1 | 0 | 0 | 0 | 33 | 1 | 1 | 179 | 87 | 13 |
| October 15 | 202 | 153 | 9 | 24 | 4 | 4 | 25 | 7 | 2 | 53 | 20 | 3 | 305 | 184 | 24 |
| October 19 | 259 | 182 | 6 | 42 | 15 | 4 | 31 | 24 | 2 | 67 | 9 | 2 | 400 | 260 | 15 |
| October 24 | 284 | 256 | 8 | 46 | 38 | 3 | 35 | 31 | 5 | 64 | 61 | 3 | 428 | 386 | 21 |
| 265 | 166 | 7 | 49 | 36 | 4 | 35 | 31 | 4 | 57 | 38 | 2 | 406 | 271 | 17 | |
| % Error | 6.7 | 35.2 | 12.5 | 6.1 | 5.3 | 25.0 | 0 | 0 | 20.0 | 10.9 | 37.7 | 33.3 | 5.1 | 29.8 | 19.0 |
| October 27 | 273 | 267 | 9 | 46 | 37 | 3 | 36 | 34 | 4 | 68 | 66 | 4 | 423 | 405 | 22 |
| November 1 | 218 | 253 | 3 | 47 | 46 | 4 | 35 | 36 | 2 | 61 | 64 | 3 | 363 | 412 | 17 |
| 216 | 177 | 4 | 50 | 38 | 3 | 36 | 13 | 1 | 60 | 50 | 3 | 362 | 278 | 11 | |
| % Error | 0.9 | 30.0 | 25.0 | 6.0 | 17.4 | 25.0 | 2.8 | 63.9 | 50.0 | 1.6 | 21.9 | 0 | 0.3 | 32.5 | 35.3 |
| November 4 | 180 | 210 | 8 | 44 | 47 | 4 | 36 | 35 | 3 | 58 | 63 | 2 | 318 | 418 | 20 |
| November 7 | 145 | 199 | 1 | 42 | 47 | 6 | 24 | 32 | 2 | 45 | 66 | 5 | 318 | 411 | 15 |
| November 16 | 34 | 118 | 6 | 15 | 38 | 7 | 2 | 101 | 2 | 3 | 28 | 2 | 59 | 395 | 18 |
| 34 | 70 | 5 | 15 | 12 | 4 | 2 | 100 | 3 | 2 | 26 | 3 | 53 | 388 | 15 | |
| % Error | 0 | 40.7 | 16.7 | 0 | 68.4 | 42.9 | 0 | 1.0 | 33.3 | 33.3 | 7.1 | 33.3 | 10.2 | 1.8 | 16.7 |
| November 26 | 4 | 30 | 3 | 1 | 16 | 3 | 4 | 121 | 1 | 1 | 97 | 1 | 10 | 329 | 13 |
| December 6 | 0 | 9 | 6 | 0 | 13 | 0 | 0 | 50 | 7 | 0 | 51 | 5 | 0 | 316 | 28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fudala, K.; Bialik, R.J. Breeding Colony Dynamics of Southern Elephant Seals at Patelnia Point, King George Island, Antarctica. Remote Sens. 2020, 12, 2964. https://doi.org/10.3390/rs12182964
Fudala K, Bialik RJ. Breeding Colony Dynamics of Southern Elephant Seals at Patelnia Point, King George Island, Antarctica. Remote Sensing. 2020; 12(18):2964. https://doi.org/10.3390/rs12182964
Chicago/Turabian StyleFudala, Katarzyna, and Robert Józef Bialik. 2020. "Breeding Colony Dynamics of Southern Elephant Seals at Patelnia Point, King George Island, Antarctica" Remote Sensing 12, no. 18: 2964. https://doi.org/10.3390/rs12182964
APA StyleFudala, K., & Bialik, R. J. (2020). Breeding Colony Dynamics of Southern Elephant Seals at Patelnia Point, King George Island, Antarctica. Remote Sensing, 12(18), 2964. https://doi.org/10.3390/rs12182964