Predicting Wheat Yield at the Field Scale by Combining High-Resolution Sentinel-2 Satellite Imagery and Crop Modelling
Abstract
:1. Introduction
2. Study Area
3. Data and Processing
3.1. Yield Data
3.2. Satellite Imagery and Atmospheric Corrections
3.3. Selection of Vegetation Indices
| Index | Equation | Reference |
|---|---|---|
| Canopy structural-related indices( VISTR) | ||
| Normalized Difference Vegetation Index (NDVI) | [44] | |
| Optimized soil-adjusted vegetation index (OSAVI) | [48] | |
| Simple ratio (SR) | [49] | |
| Difference Vegetation Index (DVI) | [44] | |
| Enhanced Vegetation Index (EVI) | [16] | |
| Enhanced Vegetation Index 2 (EVI2) | [46] | |
| Chlorophyll-related indices(VICHL) | ||
| Chlorophyll Index red edge (CIrededge) | [50] | |
| Transformed Chlorophyll Absorption in Reflectance Index (TCARI) | [51] | |
| TCARI/OSAVI (TO) | [51] | |
| Green chlorophyll vegetation index (GCVI) | [52] | |
| Green Difference Vegetation Index (GDVI) | ||
| Normalized difference red edge 1 (NDRE1) | [53] | |
| Normalized difference red edge 1 (NDRE2) | [53] | |
| Canopy Chlorophyll Content Index (CCCI) | [54] | |
4. Methods
4.1. Overview
4.2. Extraction of Canopy Development-Related Metrics
4.3. Calculation of Crop Stress Using Biophysical Model
4.4. Calculation of Crop Stress Using Thermal Imagery
4.5. Approaches for Estimating Wheat Yield at the Field Scale
4.6. Model Validation and Accuracy
5. Results
5.1. Simulated Crop Stress and Observed Yield across Seasons
5.2. Field-Scale Yield Prediction Approaches
5.2.1. Predicting Yield Using Single Canopy Development-Related Metrics
5.2.2. Predicting Wheat Yield Using Combined Model
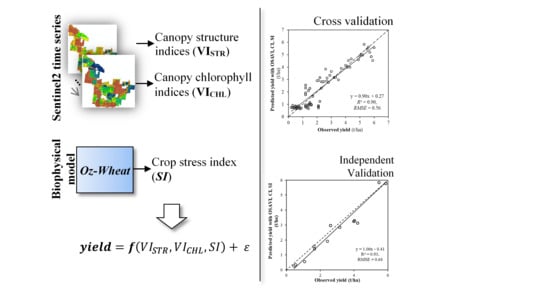
5.3. Model Calibration and Validation
5.4. Within-Field Scale Application of Combined Model
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.A.; Byerlee, D.; Edmeades, G.O. Crop Yields and Global Food Security: Will Yield Increase Continue to Feed the World; Australian Centre for International Agricultural Research: Canberra, Australia, 2014; Volume 158, p. 634.
- Potgieter, A.B.; Lobell, D.B.; Hammer, G.L.; Jordan, D.R.; Davis, P.; Brider, J. Yield trends under varying environmental conditions for sorghum and wheat across Australia. Agric. For. Meteorol. 2016, 228, 276–285. [Google Scholar] [CrossRef]
- Allan, R.J. El Niño and the Southern Oscillation: Multiscale variability and its impacts on natural ecosystems and society. In ENSO and Climatic Variability in the Last 150 Years; Diaz, H.F., Markgraf, V., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 3–55. [Google Scholar]
- Hammer, G.L. Applying seasonal climate forecasts in agricultural and natural ecosystems—A synthesis. In Applications of Seasonal Climate Forecasting in Agricultural and Natural Ecosystems—The Australian Experience; Hammer, G.L., Nicholls, N., Mitchell, C., Eds.; Atmospheric and Oceanographic Sciences Library, Kluwer: Alphen aan den Rijn, The Netherlands, 2000; Volume 21, pp. 453–462. [Google Scholar]
- Hughes, N.; Lawson, K.; Valle, H. Farm Performance and Climate: Climate-Adjusted Productivity for Broadacre Cropping Farms; Department of Agricultureand Water Resources, ABARES: Canberra, Australia, 2017; p. 61.
- Prasad, A.K.; Chai, L.; Singh, R.P.; Kafatos, M. Crop yield estimation model for Iowa using remote sensing and surface parameters. Int. J. Appl. Earth Obs. 2006, 8, 26–33. [Google Scholar] [CrossRef]
- Noureldin, N.A.; Aboelghar, M.A.; Saudy, H.S.; Ali, A.M. Rice yield forecasting models using satellite imagery in Egypt. Egypt J. Remote Sens. Space Sci. 2013, 16, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Gitelson, A.A. Remote estimation of gross primary productivity in soybean and maize based on total crop chlorophyll content. Remote Sens. Environ. 2012, 117, 440–448. [Google Scholar] [CrossRef]
- Ruecker, G.R.; Shi, Z.; Muller, M.; Conrad, C.; Ibragimov, N.; Lamers, J.P.A.; Martius, C.; Strunz, G.; Dech, S.W. Corn FPAR estimating with near and shortwave infrared bands of hyperspectral data based on PCA. In Proceedings of the International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Beijing, China, 3 July 2008. [Google Scholar]
- Pitman, J.I. Absorption of Photosynthetically Active Radiation, Radiation Use Efficiency and Spectral Reflectance of Bracken [Pteridium aquilinum (L.) Kuhn] Canopies. Ann. Bot. 2000, 85, 101–111. [Google Scholar] [CrossRef]
- Potgieter, A.B.; Hammer, G.L.; Doherty, A.; de Voil, P. Oz-Wheat: A Regional-Scale Crop Yield Simulation Model for Australian Wheat; Information Series No. QI06033; Queensland Department of Primary Industries & Fisheries: Brisbane, Australia, 2006; p. 20.
- Schut, A.G.T.; Stephens, D.J.; Stovold, R.G.H.; Adams, M.; Craig, R.L. Improved wheat yield and production forecasting with a moisture stress index, AVHRR and MODIS data. Crop Pasture Sci. 2009, 60, 60–70. [Google Scholar] [CrossRef]
- Potgieter, A.; Power, B.; Mclean, J.; Davis, P.; Rodriguez, D. Spatial estimation of wheat yields from Landsat’s visible, near infrared and thermal reflectance bands. Int. J. Remote Sens. Appl. 2014, 4, 134–143. [Google Scholar] [CrossRef]
- Lobell, D.B.; Thau, D.; Seifert, C.; Engle, E.; Little, B. A scalable satellite-based crop yield mapper. Remote Sens. Environ. 2015, 164, 324–333. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Huete, A.; Justice, C.; Liu, H. Development of vegetation and soil indices for MODIS-EOS. Remote Sens. Environ. 1994, 49, 224–234. [Google Scholar] [CrossRef]
- Huete, A.R.; Liu, H.Q.; Batchily, K.; van Leeuwen, W. A comparison of vegetation indices over a global set of TM images for EOS-MODIS. Remote Sens. Environ. 1997, 59, 440–451. [Google Scholar] [CrossRef]
- Huete, A.R.; Tucker, C.J. Investigation of soil influences in AVHRR red and near-infrared vegetation index imagery. Int. J. Remote Sens. 1991, 12, 1223–1242. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Koti, S. Canopy reflectance in cotton for growth assessment and lint yield prediction. Eur. J. Agron. 2007, 26, 335–344. [Google Scholar] [CrossRef]
- Bolton, D.K.; Friedl, M.A. Forecasting crop yield using remotely sensed vegetation indices and crop phenology metrics. Agric. For. Meteorol. 2013, 173, 74–84. [Google Scholar] [CrossRef]
- Doraiswamy, P.C.; Hatfield, J.L.; Jackson, T.J.; Akhmedov, B.; Prueger, J.; Stern, A. Crop condition and yield simulations using Landsat and MODIS. Remote Sens. Environ. 2004, 92, 548–559. [Google Scholar] [CrossRef]
- Lobell, D.B.; Asner, G.P. Regional wheat yield prediction using Landsat 7 satellite imagery. In Proceedings of the Third International Conference on Geospatial Information in Agriculture and Forestry, Denver, CO, USA, 5–7 November 2001. [Google Scholar]
- Zarco-Tejada, P.J.; Ustin, S.L.; Whiting, M.L. Temporal and Spatial Relationships between Within-Field Yield Variability in Cotton and High-Spatial Hyperspectral Remote Sensing Imagery. Agron. J. 2005, 97, 641–653. [Google Scholar] [CrossRef] [Green Version]
- Zarco-Tejada, P.J.; Berni, J.A.J.; Suárez, L.; Sepulcre-Cantó, G.; Morales, F.; Miller, J.R. Imaging chlorophyll fluorescence with an airborne narrow-band multispectral camera for vegetation stress detection. Remote Sens. Environ. 2009, 113, 1262–1275. [Google Scholar] [CrossRef]
- Potgieter, A.B.; George-Jaeggli, B.; Chapman, S.C.; Laws, K.; Suárez Cadavid, L.A.; Wixted, J.; Watson, J.; Eldridge, M.; Jordan, D.R.; Hammer, G.L. Multi-Spectral Imaging from an Unmanned Aerial Vehicle Enables the Assessment of Seasonal Leaf Area Dynamics of Sorghum Breeding Lines. Front. Plant Sci. 2017, 8, 1532. [Google Scholar] [CrossRef]
- Potgieter, A.B.; Watson, J.; Eldridge, M.; Laws, K.; George-Jaeggli, B.; Hunt, C.H.; Borrell, A.; Mace, E.; Chapman, S.C.; Jordan, D.R.; et al. Determining crop growth dynamics in sorghum breeding trials through remote and proximal sensing technologies. In Proceedings of IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; pp. 8244–8247. [Google Scholar]
- Asseng, S.; Keating, B.A.; Fillery, I.R.P.; Gregory, P.J.; Bowden, J.W.; Turner, N.C.; Palta, J.A.; Abrecht, D.G. Performance of the APSIM-wheat model in Western Australia. Field Crops Res. 1998, 57, 163–179. [Google Scholar] [CrossRef]
- Jin, X.; Kumar, L.; Li, Z.; Feng, H.; Xu, X.; Yang, G.; Wang, J. A review of data assimilation of remote sensing and crop models. Eur. J. Agron. 2018, 92, 141–152. [Google Scholar] [CrossRef]
- Jogun, T. The effect of fusing Sentinel-2 bands on land-cover classification AU—Gašparović, Mateo. Int. J. Remote Sens. 2018, 39, 822–841. [Google Scholar] [CrossRef]
- Belgiu, M.; Csillik, O. Sentinel-2 cropland mapping using pixel-based and object-based time-weighted dynamic time warping analysis. Remote Sens. Environ. 2018, 204, 509–523. [Google Scholar] [CrossRef]
- Herrmann, I.; Pimstein, A.; Karnieli, A.; Cohen, Y.; Alchanatis, V.; Bonfil, D.J. LAI assessment of wheat and potato crops by VENμS and Sentinel-2 bands. Remote Sens. Environ. 2011, 115, 2141–2151. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L.; Van den Brande, M.M.M. Using Sentinel-2 Data for Retrieving LAI and Leaf and Canopy Chlorophyll Content of a Potato Crop. Remote Sens. 2017, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Clevers, J.G.P.W.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and -3. Int. J. Appl. Earth Obs. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Al-Gaadi, K.A.; Hassaballa, A.A.; Tola, E.; Kayad, A.G.; Madugundu, R.; Alblewi, B.; Assiri, F. Prediction of Potato Crop Yield Using Precision Agriculture Techniques. PLoS ONE 2016, 11, e0162219. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.-J.; Traoré, P.C.S.; Blaes, X.; Baret, P.; Defourny, P. Estimating smallholder crops production at village level from Sentinel-2 time series in Mali’s cotton belt. Remote Sens. Environ. 2018, 216, 647–657. [Google Scholar] [CrossRef]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, J.L. Potato Yield Prediction Using Machine Learning Techniques and Sentinel 2 Data. Remote Sens. 2019, 11, 1745. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Mostovoy, G. Cotton Yield Estimate Using Sentinel-2 Data and an Ecosystem Model over the Southern US. Remote Sens.-Basel 2019, 11, 2000. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.A. Wheat physiology: A review of recent developments. Crop Pasture Sci. 2011, 62, 95–114. [Google Scholar] [CrossRef] [Green Version]
- Vermote, E.F.; Tanre, D.; Deuze, J.L.; Herman, M.; Morcette, J. Second Simulation of the Satellite Signal in the Solar Spectrum, 6S: An overview. IEEE Trans. Geosci. Remote Sens. 1997, 35, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.T. Py6S: A Python interface to the 6S radiative transfer model. Comput. Geosci.-UK 2013, 51, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S. Atmospheric Correction of a (Single) Sentinel 2 Image. Available online: https://github.com/samsammurphy/gee-atmcorr-S2 (accessed on 19 March 2020).
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Steven, M.D. The Sensitivity of the OSAVI Vegetation Index to Observational Parameters. Remote Sens. Environ. 1998, 63, 49–60. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus hippocastanum L. and Acer platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D. Coincident detection of crop water stress, nitrogen status and canopy density using ground-based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Potgieter, A.B.; Hammer, G.L.; Doherty, A.; de Voil, P. A simple regional-scale model for forecasting sorghum yield across North-Eastern Australia. Agric. For. Meteorol. 2005, 132, 143–153. [Google Scholar] [CrossRef]
- Fitzpatrick, E.A.; Nix, H.A. A model for simulating soil water regime in alternating fallow-crop systems. Agric. Meteorol. 1969, 6, 303–319. [Google Scholar] [CrossRef]
- Ritchie, J.T. Model for predicting evaporation from a row crop with incomplete cover. Water Resour. Res. 1972, 8, 1204–1213. [Google Scholar] [CrossRef] [Green Version]
- Nix, H.A.; Fitzpatrick, E.A. An index of crop water stress related to wheat and grain sorghum yields. Agric. Meteorol. 1969, 6, 321–337. [Google Scholar] [CrossRef]
- Keating, B.A.; Meinke, H. Assessing exceptional drought with a cropping systems simulator: A case study for grain production in northeast Australia. Agric. Syst. 1998, 57, 315–332. [Google Scholar] [CrossRef]
- Innes, P.J.; Tan, D.K.Y.; Van Ogtrop, F.; Amthor, J.S. Effects of high-temperature episodes on wheat yields in New South Wales, Australia. Agric. For. Meteorol. 2015, 208, 95–107. [Google Scholar] [CrossRef]
- Thapa, S.; Jessup, K.E.; Pradhan, G.P.; Rudd, J.C.; Liu, S.; Mahan, J.R.; Devkota, R.N.; Baker, J.A.; Xue, Q. Canopy temperature depression at grain filling correlates to winter wheat yield in the U.S. Southern High Plains. Field Crops Res. 2018, 217, 11–19. [Google Scholar] [CrossRef]
- Gillespie, A. Land Surface Emissivity. In Encyclopedia of Remote Sensing; Njoku, E.G., Ed.; Springer New York: New York, NY, USA, 2014; pp. 303–311. [Google Scholar] [CrossRef]
- Maindonald, J.H.; Braun, W.J. Package ‘GAAG’. Available online: https://cran.r-project.org/web/packages/DAAG/DAAG.pdf (accessed on 19 March 2020).
- Haboudane, D.; Tremblay, N.; Miller, J.R.; Vigneault, P. Remote Estimation of Crop Chlorophyll Content Using Spectral Indices Derived From Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2008, 46, 423–437. [Google Scholar] [CrossRef]
- Wolanin, A.; Camps-Valls, G.; Gómez-Chova, L.; Mateo-García, G.; van der Tol, C.; Zhang, Y.; Guanter, L. Estimating crop primary productivity with Sentinel-2 and Landsat 8 using machine learning methods trained with radiative transfer simulations. Remote Sens. Environ. 2019, 225, 441–457. [Google Scholar] [CrossRef]
- Christopher, J.; Christopher, M.J.; Borrell, A.K.; Fletcher, S.; Chenu, K. Stay-green traits to improve wheat adaptation in well-watered and water-limited environments. J. Exp. Bot. 2016, 67, 5159–5172. [Google Scholar] [CrossRef] [Green Version]
- Knox, J.; Hess, T.; Daccache, A.; Wheeler, T. Climate change impacts on crop productivity in Africa and South Asia. Environ. Res. Lett. 2012, 7, 034032. [Google Scholar] [CrossRef]
- Delmotte, S.; Tittonell, P.; Mouret, J.C.; Hammond, R.; Lopez-Ridaura, S. On farm assessment of rice yield variability and productivity gaps between organic and conventional cropping systems under Mediterranean climate. Eur. J. Agron. 2011, 35, 223–236. [Google Scholar] [CrossRef]
- Hunt, M.L.; Blackburn, G.A.; Carrasco, L.; Redhead, J.W.; Rowland, C.S. High resolution wheat yield mapping using Sentinel-2. Remote Sens. Environ. 2019, 233, 111410. [Google Scholar] [CrossRef]
- Evans, L.T. Crop Evolution, Adaptation and Yield; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Fischer, R.A. Number of kernels in wheat crops and the influence of solar radiation and temperature. J. Agric. Sci. 1985, 105, 447–461. [Google Scholar] [CrossRef]







| Cropping Season | Date/Month |
|---|---|
| Calibration | |
| 2016 | 14/01, 23/02, 14/03, 13/04, 13/05, 02/06, 01/08, 21/08, 10/09, 20/10, 19/11, 09/12, 19/12, 29/12 |
| 2017 | 18/01, 07/02, 17/02, 09/03, 18/04, 08/05, 28/05, 17/06, 02/07, 22/07, 27/07, 11/08, 16/08, 31/08, 05/09, 20/09, 10/10, 30/10, 04/11, 09/11, 24/11, 09/12, 14/12, 19/12, 29/12 |
| Validation | |
| 2016 | 13/05, 01/08, 21/08, 20/10 |
| 2017 | 02/07, 22/07, 27/07, 11/08, 16/08, 31/08, 05/09, 20/09 |
| Index | R2 | RMSE (t/ha) | p | Index | R2 | RMSE (t/ha) | p |
|---|---|---|---|---|---|---|---|
| Canopy structural-related indices | Chlorophyll-related indices | ||||||
| PeakOSAVI | 0.74 | 0.91 | 5.81 × 10−27 | PeakCI | 0.76 | 0.88 | 2.55 × 10−28 |
| PeakNDVI | 0.74 | 0.91 | 8.11 × 10−27 | PeakNDRE1 | 0.76 | 0.88 | 2.83 × 10−28 |
| PeakSR | 0.73 | 0.92 | 2.07 × 10−26 | PeakNDRE2 | 0.74 | 0.91 | 4.43 × 10−27 |
| PeakEVI2 | 0.72 | 0.93 | 5.76 × 10−26 | PeakGCVI | 0.72 | 0.93 | 4.93 × 10−26 |
| PeakEVI | 0.72 | 0.94 | 8.19 × 10−26 | PeakTCARI | 0.55 | 1.19 | 1.20 × 10−16 |
| PeakDVI | 0.70 | 0.96 | 9.69 × 10−25 | PeakGDVI | 0.68 | 0.99 | 1.55 × 10−23 |
| PeakTO | 0.34 | 1.44 | 1.70 × 10−09 | ||||
| Crop development metrics | |||||||
| Green up rate | 0.49 | 1.22 | 1.26 × 10−13 | ||||
| Senescence rate | 0.56 | 1.14 | 4.05 × 10−16 | ||||
| Index Combinations | R2 | RMSE (t/ha) | p |
|---|---|---|---|
| Canopy structural-related index + chlorophyll-related index | |||
| PeakOSAVI + PeakCI | 0.77 | 0.86 | 2.56 × 10−28 |
| PeakOSAVI + PeakNDRE1 | 0.76 | 0.86 | 4.75 × 10−28 |
| PeakNDVI + PeakCI | 0.78 | 0.84 | 6.82 × 10−29 |
| PeakNDVI + PeakNDRE1 | 0.76 | 0.88 | 1.05 × 10−27 |
| Canopy structural-related index + chlorophyll-related index + SI (or LST) | |||
| PeakOSAVI + PeakCI + SI | 0.91 | 0.54 | 2.10 × 10−42 |
| PeakOSAVI + PeakNDRE1 + SI | 0.86 | 0.66 | 8.53 × 10−36 |
| PeakNDVI + PeakCI + SI | 0.91 | 0.54 | 3.80 × 10−42 |
| PeakNDVI + PeakNDRE1 + SI | 0.88 | 0.61 | 2.54 × 10−38 |
| PeakOSAVI + PeakCI + LSTmax | 0.85 | 0.71 | 5.68 × 10−29 |
| PeakOSAVI + PeakCI + LSTmean | 0.69 | 1.04 | 1.87 × 10−19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Potgieter, A.B.; Zhang, M.; Wu, B.; Hammer, G.L. Predicting Wheat Yield at the Field Scale by Combining High-Resolution Sentinel-2 Satellite Imagery and Crop Modelling. Remote Sens. 2020, 12, 1024. https://doi.org/10.3390/rs12061024
Zhao Y, Potgieter AB, Zhang M, Wu B, Hammer GL. Predicting Wheat Yield at the Field Scale by Combining High-Resolution Sentinel-2 Satellite Imagery and Crop Modelling. Remote Sensing. 2020; 12(6):1024. https://doi.org/10.3390/rs12061024
Chicago/Turabian StyleZhao, Yan, Andries B Potgieter, Miao Zhang, Bingfang Wu, and Graeme L Hammer. 2020. "Predicting Wheat Yield at the Field Scale by Combining High-Resolution Sentinel-2 Satellite Imagery and Crop Modelling" Remote Sensing 12, no. 6: 1024. https://doi.org/10.3390/rs12061024
APA StyleZhao, Y., Potgieter, A. B., Zhang, M., Wu, B., & Hammer, G. L. (2020). Predicting Wheat Yield at the Field Scale by Combining High-Resolution Sentinel-2 Satellite Imagery and Crop Modelling. Remote Sensing, 12(6), 1024. https://doi.org/10.3390/rs12061024