Crop Phenology Modelling Using Proximal and Satellite Sensor Data
Abstract
:1. Introduction
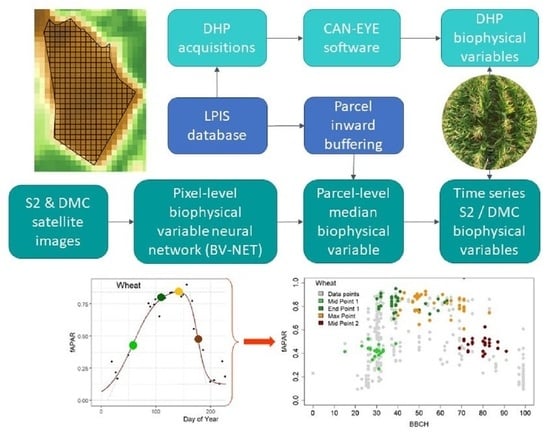
2. Materials and Methods
2.1. Satellite Sensor Observations
2.2. Digital Hemispherical Photography and Ground Observations
2.3. Statistical Modelling Methods
3. Results
3.1. Digital Hemispherical Photography and Ground Observations
3.2. Satellite Observations
3.3. Differences and Similarities between Sensors during the Cropping Season
3.4. Crop Phenology Detection Differences and Similarities between Sensors during the Cropping Season
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craufurd, P.Q.; Wheeler, T.R. Climate Change and the Flowering Time of Annual Crops. J. Exp. Bot. 2009, 60, 2529–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmielewski, F.-M.; Müller, A.; Bruns, E. Climate Changes and Trends in Phenology of Fruit Trees and Field Crops in Germany, 1961–2000. Agric. For. Meteorol. 2004, 121, 69–78. [Google Scholar] [CrossRef]
- Eyshi Rezaei, E.; Siebert, S.; Ewert, F. Climate and Management Interaction Cause Diverse Crop Phenology Trends. Agric. For. Meteorol. 2017, 233, 55–70. [Google Scholar] [CrossRef]
- Menzel, A.; Yuan, Y.; Matiu, M.; Sparks, T.; Scheifinger, H.; Gehrig, R.; Estrella, N. Climate Change Fingerprints in Recent European Plant Phenology. Glob. Chang. Biol. 2020, 26, 2599–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damien, M.; Tougeron, K. Prey–Predator Phenological Mismatch under Climate Change. Curr. Opin. Insect Sci. 2019, 35, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, A.; Caffarra, A.; O’Neill, B.F. A Review of Climate-Driven Mismatches between Interdependent Phenophases in Terrestrial and Aquatic Ecosystems. Int. J. Biometeorol. 2011, 55, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Eyshi Rezaei, E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. Heat Stress in Cereals: Mechanisms and Modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Gobin, A. Weather Related Risks in Belgian Arable Agriculture. Agric. Syst. 2018, 159, 225–236. [Google Scholar] [CrossRef]
- Gobin, A.; Van de Vyver, H. Spatio-Temporal Variability of Dry and Wet Spells and Their Influence on Crop Yields. Agric. For. Meteorol. 2021, 308–309, 108565. [Google Scholar] [CrossRef]
- Drepper, B.; Gobin, A.; Van Orshoven, J. Spatio-Temporal Assessment of Frost Risks during the Flowering of Pear Trees in Belgium for 1971–2068. Agric. For. Meteorol. 2022, 315, 108822. [Google Scholar] [CrossRef]
- Tolomio, M.; Casa, R. Dynamic Crop Models and Remote Sensing Irrigation Decision Support Systems: A Review of Water Stress Concepts for Improved Estimation of Water Requirements. Remote Sens. 2020, 12, 3945. [Google Scholar] [CrossRef]
- Drepper, B.; Bamps, B.; Gobin, A.; Van Orshoven, J. Strategies for Managing Spring Frost Risks in Orchards: Effectiveness and Conditionality—A Systematic Review Protocol. Environ. Evid. 2021, 10, 32. [Google Scholar] [CrossRef]
- Kostková, M.; Hlavinka, P.; Pohanková, E.; Kersebaum, K.C.; Nendel, C.; Gobin, A.; Olesen, J.E.; Ferrise, R.; Dibari, C.; Takáč, J.; et al. Performance of 13 Crop Simulation Models and Their Ensemble for Simulating Four Field Crops in Central Europe. J. Agric. Sci. 2021, 159, 69–89. [Google Scholar] [CrossRef]
- Seidel, S.J.; Palosuo, T.; Thorburn, P.; Wallach, D. Towards Improved Calibration of Crop Models—Where Are We Now and Where Should We Go? Eur. J. Agron. 2018, 94, 25–35. [Google Scholar] [CrossRef]
- Kersebaum, K.; Kroes, J.; Gobin, A.; Takáč, J.; Hlavinka, P.; Trnka, M.; Ventrella, D.; Giglio, L.; Ferrise, R.; Moriondo, M.; et al. Assessing Uncertainties of Water Footprints Using an Ensemble of Crop Growth Models on Winter Wheat. Water 2016, 8, 571. [Google Scholar] [CrossRef] [Green Version]
- Asseng, S.; Ewert, F.; Rosenzweig, C.; Jones, J.W.; Hatfield, J.L.; Ruane, A.C.; Boote, K.J.; Thorburn, P.J.; Rötter, R.P.; Cammarano, D.; et al. Uncertainty in Simulating Wheat Yields under Climate Change. Nat. Clim. Change 2013, 3, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Bassu, S.; Brisson, N.; Durand, J.-L.; Boote, K.; Lizaso, J.; Jones, J.W.; Rosenzweig, C.; Ruane, A.C.; Adam, M.; Baron, C.; et al. How Do Various Maize Crop Models Vary in Their Responses to Climate Change Factors? Glob. Change Biol. 2014, 20, 2301–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jägermeyr, J.; Müller, C.; Ruane, A.C.; Elliott, J.; Balkovic, J.; Castillo, O.; Faye, B.; Foster, I.; Folberth, C.; Franke, J.A.; et al. Climate Impacts on Global Agriculture Emerge Earlier in New Generation of Climate and Crop Models. Nat. Food 2021, 2, 873–885. [Google Scholar] [CrossRef]
- Liu, L.; Wallach, D.; Li, J.; Liu, B.; Zhang, L.; Tang, L.; Zhang, Y.; Qiu, X.; Cao, W.; Zhu, Y. Uncertainty in Wheat Phenology Simulation Induced by Cultivar Parameterization under Climate Warming. Eur. J. Agron. 2018, 94, 46–53. [Google Scholar] [CrossRef]
- Wajid, A.; Hussain, K.; Ilyas, A.; Habib-ur-Rahman, M.; Shakil, Q.; Hoogenboom, G. Crop Models: Important Tools in Decision Support System to Manage Wheat Production under Vulnerable Environments. Agriculture 2021, 11, 1166. [Google Scholar] [CrossRef]
- Raymundo, R.; Asseng, S.; Cammarano, D.; Quiroz, R. Potato, Sweet Potato, and Yam Models for Climate Change: A Review. Field Crops Res. 2014, 166, 173–185. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yokozawa, M.; Toritani, H.; Shibayama, M.; Ishitsuka, N.; Ohno, H. A Crop Phenology Detection Method Using Time-Series MODIS Data. Remote Sens. Environ. 2005, 96, 366–374. [Google Scholar] [CrossRef]
- Bolton, D.K.; Gray, J.M.; Melaas, E.K.; Moon, M.; Eklundh, L.; Friedl, M.A. Continental-Scale Land Surface Phenology from Harmonized Landsat 8 and Sentinel-2 Imagery. Remote Sens. Environ. 2020, 240, 111685. [Google Scholar] [CrossRef]
- Durgun, Y.; Gobin, A.; Van De Kerchove, R.; Tychon, B. Crop Area Mapping Using 100-m Proba-V Time Series. Remote Sens. 2016, 8, 585. [Google Scholar] [CrossRef] [Green Version]
- Durgun, Y.Ö.; Gobin, A.; Duveiller, G.; Tychon, B. A Study on Trade-Offs between Spatial Resolution and Temporal Sampling Density for Wheat Yield Estimation Using Both Thermal and Calendar Time. Int. J. Appl. Earth Obs. Geoinf. 2020, 86, 101988. [Google Scholar] [CrossRef]
- Atzberger, C. Advances in Remote Sensing of Agriculture: Context Description, Existing Operational Monitoring Systems and Major Information Needs. Remote Sens. 2013, 5, 949–981. [Google Scholar] [CrossRef] [Green Version]
- Misra, G.; Cawkwell, F.; Wingler, A. Status of Phenological Research Using Sentinel-2 Data: A Review. Remote Sens. 2020, 12, 2760. [Google Scholar] [CrossRef]
- Rivas, H.; Delbart, N.; Ottlé, C.; Maignan, F.; Vaudour, E. Disaggregated PROBA-V Data Allows Monitoring Individual Crop Phenology at a Higher Observation Frequency than Sentinel-2. Int. J. Appl. Earth Obs. Geoinf. 2021, 104, 102569. [Google Scholar] [CrossRef]
- Zeng, L.; Wardlow, B.D.; Xiang, D.; Hu, S.; Li, D. A Review of Vegetation Phenological Metrics Extraction Using Time-Series, Multispectral Satellite Data. Remote Sens. Environ. 2020, 237, 111511. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Atzberger, C.; Høgda, K.A.; Johansen, B.; Skidmore, A.K. Improved Monitoring of Vegetation Dynamics at Very High Latitudes: A New Method Using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Gao, F.; Anderson, M.; Daughtry, C.; Karnieli, A.; Hively, D.; Kustas, W. A Within-Season Approach for Detecting Early Growth Stages in Corn and Soybean Using High Temporal and Spatial Resolution Imagery. Remote Sens. Environ. 2020, 242, 111752. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring Vegetation Phenology Using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Gao, F.; Anderson, M.C.; Zhang, X.; Yang, Z.; Alfieri, J.G.; Kustas, W.P.; Mueller, R.; Johnson, D.M.; Prueger, J.H. Toward Mapping Crop Progress at Field Scales through Fusion of Landsat and MODIS Imagery. Remote Sens. Environ. 2017, 188, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Duveiller, G.; Weiss, M.; Baret, F.; Defourny, P. Retrieving Wheat Green Area Index during the Growing Season from Optical Time Series Measurements Based on Neural Network Radiative Transfer Inversion. Remote Sens. Environ. 2011, 115, 887–896. [Google Scholar] [CrossRef]
- Koetz, B.; Baret, F.; Poilvé, H.; Hill, J. Use of Coupled Canopy Structure Dynamic and Radiative Transfer Models to Estimate Biophysical Canopy Characteristics. Remote Sens. Environ. 2005, 95, 115–124. [Google Scholar] [CrossRef]
- Vannoppen, A.; Gobin, A.; Kotova, L.; Top, S.; De Cruz, L.; Vīksna, A.; Aniskevich, S.; Bobylev, L.; Buntemeyer, L.; Caluwaerts, S.; et al. Wheat Yield Estimation from NDVI and Regional Climate Models in Latvia. Remote Sens. 2020, 12, 2206. [Google Scholar] [CrossRef]
- Vannoppen, A.; Gobin, A. Estimating Farm Wheat Yields from NDVI and Meteorological Data. Agronomy 2021, 11, 946. [Google Scholar] [CrossRef]
- Vannoppen, A.; Gobin, A. Estimating Yield from NDVI, Weather Data, and Soil Water Depletion for Sugar Beet and Potato in Northern Belgium. Water 2022, 14, 1188. [Google Scholar] [CrossRef]
- De Keukelaere, L.; Sterckx, S.; Adriaensen, S.; Knaeps, E.; Reusen, I.; Giardino, C.; Bresciani, M.; Hunter, P.; Neil, C.; Van der Zande, D.; et al. Atmospheric Correction of Landsat-8/OLI and Sentinel-2/MSI Data Using ICOR Algorithm: Validation for Coastal and Inland Waters. Eur. J. Remote Sens. 2018, 51, 525–542. [Google Scholar] [CrossRef] [Green Version]
- Irish, R.R.; Barker, J.L.; Goward, S.N.; Arvidson, T. Characterization of the Landsat-7 ETM+ Automated Cloud-Cover Assessment (ACCA) Algorithm. Photogramm. Eng. Remote Sens. 2006, 72, 1179–1188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guindon, B.; Cihlar, J. An Image Transform to Characterize and Compensate for Spatial Variations in Thin Cloud Contamination of Landsat Images. Remote Sens. Environ. 2002, 82, 173–187. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; Myneni, R.B.; Pragnère, A.; Knyazikhin, Y. Investigation of a Model Inversion Technique to Estimate Canopy Biophysical Variables from Spectral and Directional Reflectance Data. Agronomie 2000, 20, 3–22. [Google Scholar] [CrossRef]
- Baret, F.; Hagolle, O.; Geiger, B.; Bicheron, P.; Miras, B.; Huc, M.; Berthelot, B.; Niño, F.; Weiss, M.; Samain, O.; et al. LAI, FAPAR and FCover CYCLOPES Global Products Derived from VEGETATION. Remote Sens. Environ. 2007, 110, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Claverie, M.; Vermote, E.F.; Weiss, M.; Baret, F.; Hagolle, O.; Demarez, V. Validation of Coarse Spatial Resolution LAI and FAPAR Time Series over Cropland in Southwest France. Remote Sens. Environ. 2013, 139, 216–230. [Google Scholar] [CrossRef]
- Li, W.; Weiss, M.; Waldner, F.; Defourny, P.; Demarez, V.; Morin, D.; Hagolle, O.; Baret, F. A Generic Algorithm to Estimate LAI, FAPAR and FCOVER Variables from SPOT4_HRVIR and Landsat Sensors: Evaluation of the Consistency and Comparison with Ground Measurements. Remote Sens. 2015, 7, 15494–15516. [Google Scholar] [CrossRef] [Green Version]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT+SAIL Models: A Review of Use for Vegetation Characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. S2ToolBox Level 2 Products: LAI, FAPAR, FCOVER. Version 1.1. 2016. Available online: http://step.esa.int/docs/extra/ATBD_S2ToolBox_L2B_V1.1.pdf (accessed on 8 April 2023).
- Vuolo, F.; Żółtak, M.; Pipitone, C.; Zappa, L.; Wenng, H.; Immitzer, M.; Weiss, M.; Baret, F.; Atzberger, C. Data Service Platform for Sentinel-2 Surface Reflectance and Value-Added Products: System Use and Examples. Remote Sens. 2016, 8, 938. [Google Scholar] [CrossRef] [Green Version]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heß, M.; Lancashire, P.D.; Schnock, U.; Stauß, R.; Van Den Boom, T.; et al. The BBCH System to Coding the Phenological Growth Stages of Plants–History and Publications. J. Für. Kult. 2009, 61, 41–52. [Google Scholar]
- Delloye, C.; Weiss, M.; Defourny, P. Retrieval of the Canopy Chlorophyll Content from Sentinel-2 Spectral Bands to Estimate Nitrogen Uptake in Intensive Winter Wheat Cropping Systems. Remote Sens. Environ. 2018, 216, 245–261. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. CAN-EYE V6.1 User Manual. 2010. EMMAH Laboratory (Mediterranean Environment and Agro-Hydro System Modelisation). French National Institute of Agricultural Research (INRA). 2010. Available online: http://jecam.org/wp-content/uploads/2018/07/CAN_EYE_User_Manual.pdf (accessed on 8 April 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Zambrano-Bigiarini, M. HydroGOF: Goodness-of-Fit Functions for Comparison of Simulated and Observed Hydrological Time Series. R Package Version 0.3-2. 2011. Available online: http://cran.r-project.org/web/packages/hydroGOF/hydroGOF.pdf (accessed on 8 April 2023).
- Perondi, D.; Fraisse, C.W.; Staub, C.G.; Cerbaro, V.A.; Barreto, D.D.; Pequeno, D.N.L.; Mulvaney, M.J.; Troy, P.; Pavan, W. Crop Season Planning Tool: Adjusting Sowing Decisions to Reduce the Risk of Extreme Weather Events. Comput. Electron. Agric. 2019, 156, 62–70. [Google Scholar] [CrossRef]
- Divya, K.L.; Mhatre, P.H.; Venkatasalam, E.P.; Sudha, R. Crop Simulation Models as Decision-Supporting Tools for Sustainable Potato Production: A Review. Potato Res. 2021, 64, 387–419. [Google Scholar] [CrossRef]
- Post, A.K.; Hufkens, K.; Richardson, A.D. Predicting Spring Green-up across Diverse North American Grasslands. Agric. For. Meteorol. 2022, 327, 109204. [Google Scholar] [CrossRef]
- Minet, J.; Curnel, Y.; Gobin, A.; Goffart, J.-P.; Melard, F.; Tychon, B.; Wellens, J.; Defourny, P. Crowdsourcing for Agricultural Applications: A Review of Uses and Opportunities for a Farmsourcing Approach. Comput. Electron. Agric. 2017, 142, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Durgun, Y.; Gobin, A.; Gilliams, S.; Duveiller, G.; Tychon, B. Testing the Contribution of Stress Factors to Improve Wheat and Maize Yield Estimations Derived from Remotely-Sensed Dry Matter Productivity. Remote Sens. 2016, 8, 170. [Google Scholar] [CrossRef] [Green Version]
- Baetens, L.; Desjardins, C.; Hagolle, O. Validation of Copernicus Sentinel-2 Cloud Masks Obtained from MAJA, Sen2Cor, and FMask Processors Using Reference Cloud Masks Generated with a Supervised Active Learning Procedure. Remote Sens. 2019, 11, 433. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Miao, Y.; Gupta, S.K.; Rosen, C.J.; Yuan, F.; Wang, C.; Wang, L.; Huang, Y. Improving Potato Yield Prediction by Combining Cultivar Information and UAV Remote Sensing Data Using Machine Learning. Remote Sens. 2021, 13, 3322. [Google Scholar] [CrossRef]
- Van Tricht, K.; Gobin, A.; Gilliams, S.; Piccard, I. Synergistic Use of Radar Sentinel-1 and Optical Sentinel-2 Imagery for Crop Mapping: A Case Study for Belgium. Remote Sens. 2018, 10, 1642. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fang, S.; Zhao, L.; Huang, X.; Jiang, X. Parcel-Based Summer Maize Mapping and Phenology Estimation Combined Using Sentinel-2 and Time Series Sentinel-1 Data. Int. J. Appl. Earth Obs. Geoinf. 2022, 108, 102720. [Google Scholar] [CrossRef]
- De Grave, C.; Verrelst, J.; Morcillo-Pallarés, P.; Pipia, L.; Rivera-Caicedo, J.P.; Amin, E.; Belda, S.; Moreno, J. Quantifying Vegetation Biophysical Variables from the Sentinel-3/FLEX Tandem Mission: Evaluation of the Synergy of OLCI and FLORIS Data Sources. Remote Sens. Environ. 2020, 251, 112101. [Google Scholar] [CrossRef]
- Gobin, A. Modelling Climate Impacts on Crop Yields in Belgium. Clim. Res. 2010, 44, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Gobin, A. Impact of Heat and Drought Stress on Arable Crop Production in Belgium. Nat. Hazards Earth Syst. Sci. 2012, 12, 1911–1922. [Google Scholar] [CrossRef] [Green Version]


















| Crop | Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Winter wheat | f | f | h | h | s | s | ||||||||||
| Silage maize | s | s | e | f | f | h | h | |||||||||
| Late potato | p | p | e | e | t | t | h | h | ||||||||
| 2015 DMC (22 m) | 3 | 2 | 2 | 2 | 1 | |||||||||||
| 2016 DMC (22 m) | 3 | 2 | 2 | 4 | 2 | 4 | 4 | |||||||||
| 2015 Sentinel-2A (10 m) | 1 | 2 | 4 | 4 | 3 | 1 | 1 | |||||||||
| 2016 Sentinel-2A (10 m) | 5 | 4 | 5 | 4 | 2 | 1 | 4 | 5 | 3 | 4 | 4 | 5 | 4 | 5 | 4 | 4 |
| 2017 Sentinel-2A&B (10 m) | 3 | 6 | 3 | 4 | 3 | 5 | 7 | 8 | 5 | 8 | 5 | 9 | 5 | 9 | 5 | 6 |
| Crop | 2015 | 2016 | 2017 | Total |
|---|---|---|---|---|
| Silage Maize | 10 (10) | 20 (09) | 69 (15) | 99 (34) |
| Late Potato | 20 (01) | 29 (05) | 28 (06) | 77 (12) |
| Winter wheat | 37 (19) | 22 (09) | 63 (10) | 122 (38) |
| Crop | Variable | MAE | RMSE | R2 | d |
|---|---|---|---|---|---|
| Silage Maize | fAPAR fCover | 0.03–0.08 0.01–0.09 | 0.03–0.09 0.01–0.10 | 0.75–0.99 0.75–0.99 | 0.93–0.99 0.93–0.99 |
| Late Potato | fAPAR fCover | 0.00–0.11 0.00–0.14 | 0.00–0.13 0.00–0.15 | 0.46–0.99 0.55–0.99 | 0.81–0.99 0.50–0.99 |
| Winter wheat | fAPAR fCover | 0.00–0.09 0.00–0.18 | 0.00–0.09 0.00–0.24 | 0.60–0.99 0.43–0.99 | 0.53–0.99 0.82–0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobin, A.; Sallah, A.-H.M.; Curnel, Y.; Delvoye, C.; Weiss, M.; Wellens, J.; Piccard, I.; Planchon, V.; Tychon, B.; Goffart, J.-P.; et al. Crop Phenology Modelling Using Proximal and Satellite Sensor Data. Remote Sens. 2023, 15, 2090. https://doi.org/10.3390/rs15082090
Gobin A, Sallah A-HM, Curnel Y, Delvoye C, Weiss M, Wellens J, Piccard I, Planchon V, Tychon B, Goffart J-P, et al. Crop Phenology Modelling Using Proximal and Satellite Sensor Data. Remote Sensing. 2023; 15(8):2090. https://doi.org/10.3390/rs15082090
Chicago/Turabian StyleGobin, Anne, Abdoul-Hamid Mohamed Sallah, Yannick Curnel, Cindy Delvoye, Marie Weiss, Joost Wellens, Isabelle Piccard, Viviane Planchon, Bernard Tychon, Jean-Pierre Goffart, and et al. 2023. "Crop Phenology Modelling Using Proximal and Satellite Sensor Data" Remote Sensing 15, no. 8: 2090. https://doi.org/10.3390/rs15082090
APA StyleGobin, A., Sallah, A. -H. M., Curnel, Y., Delvoye, C., Weiss, M., Wellens, J., Piccard, I., Planchon, V., Tychon, B., Goffart, J. -P., & Defourny, P. (2023). Crop Phenology Modelling Using Proximal and Satellite Sensor Data. Remote Sensing, 15(8), 2090. https://doi.org/10.3390/rs15082090