Whey Protein Ingestion Activates mTOR-dependent Signalling after Resistance Exercise in Young Men: A Double-Blinded Randomized Controlled Trial
Abstract
:1. Introduction
2. Results and Discussion
2.1. Resistance Exercise
| Placebo | WPI | |
|---|---|---|
| (n = 7) | (n = 7) | |
| Age, yr | 23.0 ± 0.9 | 21.9 ± 0.8 |
| Height, cm | 178.0 ± 3.5 | 176.7 ± 2.7 |
| Mass, kg | 76.7 ± 4.5 | 76.2 ± 2.8 |
| BMI, kg·m2 | 24.1 ± 0.1 | 24.5 ± 1.1 |
| Peak Torque (Nm) | ||
| Concentric | 212.3 ± 13.3 | 213.1 ± 10.9 |
| Eccentric | 260.7 ± 16.2 | 255.7 ± 9.9 |
2.2. Amino Acid Analysis of Supplements
| Amino acid g / 200 mL | WPI drink |
|---|---|
| Essential amino acids | |
| Histidine | 0.56 |
| Isoleucine | 1.49 |
| Lysine | 2.87 |
| Methionine | 0.71 |
| Phenylalanine | 0.93 |
| Threonine | 1.30 |
| Leucine | 3.66 |
| Valine | 1.41 |
| Nonessential amino acids | |
| Alanine | 1.57 |
| Arginine | 0.73 |
| Aspartate | 2.94 |
| Glutamate | 4.80 |
| Glycine | 0.39 |
| Proline | 1.30 |
| Serine | 1.01 |
| Tyrosine | 0.95 |
| Total Amino Acids (g) | 26.6 |
| Total BCAA | 6.6 |
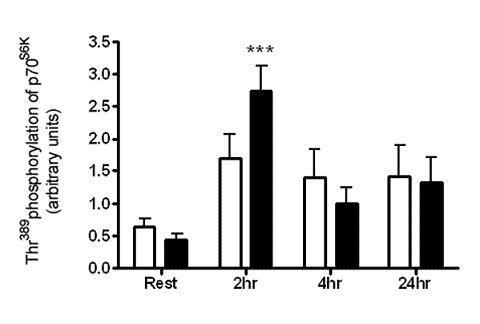
2.3. Kinase Analysis





3. Experimental Section
3.1. Subjects
3.2. Nutritional Composition of Supplement Drinks
3.3. Amino Acid Analysis of Supplement Drinks
3.4. Experimental Design
3.5. Tissue Processing
3.6. Immunoblot Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273, 99–107. [Google Scholar]
- Miller, B.F.; Olesen, J.L.; Hansen, M.; Dossing, S.; Crameri, R.M.; Welling, R.J.; Langberg, H.; Flyvbjerg, A.; Kjaer, M.; Babraj, J.A. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J. Physiol. 2005, 567, 1021–1033. [Google Scholar]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kubica, N.; Bolster, D.R.; Farrell, P.A.; Kimball, S.R.; Jefferson, L.S. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2B {epsilon} mRNA in a mammalian target of rapamycin-dependent manner. J. Biol. Chem. 2005, 280, 7570. [Google Scholar]
- Liu, Z.; Jahn, L.A.; Wei, L.; Long, W.; Barrett, E.J. Amino acids stimulate translation initiation and protein synthesis through an Akt-Independent pathway in human skeletal muscle. J. Clin. Endocrinol. Metab. 2002, 87, 5553–5558. [Google Scholar]
- Karlsson, H.K.R.; Nilsson, P.A.; Nilsson, J.; Chibalin, A.V.; Zierath, J.R.; Blomstrand, E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 287, 1–7. [Google Scholar]
- Blomstrand, E.; Eliasson, J.; Karlsson, H.K.; Kohnke, R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J. Nutr. 2006, 136, 269S–273S. [Google Scholar]
- Nave, B.T.; Ouwens, D.M.; Withers, D.J.; Alessi, D.R.; Shepherd, P.R. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 1999, 344, 427–431. [Google Scholar]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Wolf, S.E.; Sanford, A.P.; Wolfe, R.R. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med. Sci. Sports Exerc. 2004, 36, 2073. [Google Scholar]
- Petróczi, A.; Naughton, D.P.; Mazanov, J.; Holloway, A.; Bingham, J. Performance enhancement with supplements: incongruence between rationale and practice. J. Int. Soc. Sports Nutr. 2007, 4, 19. [Google Scholar]
- Froiland, K.; Koszewski, W.; Hingst, J.; Kopecky, L. Nutritional supplement use among college athletes and their sources of information. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 104–120. [Google Scholar]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Aarsland, A.A.; Sanford, A.P.; Wolfe, R.R. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E71. [Google Scholar]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI (3)/Akt/mTOR and PI (3) K/Akt/GSK 3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar]
- Farrell, P.A.; Fedele, M.J.; Vary, T.C.; Kimball, S.R.; Lang, C.H.; Jefferson, L.S. Regulation of protein synthesis after acute resistance exercise in diabetic rats. Am. J. Physiol. Endocrinol. Metab. 1999, 276, 721–727. [Google Scholar]
- Vyas, D.R.; Spangenburg, E.E.; Abraha, T.W.; Childs, T.E.; Booth, F.W. GSK-3ß negatively regulates skeletal myotube hypertrophy. Am. J. Physiol. Cell Physiol. 2002, 283, 545–551. [Google Scholar]
- Lai, K.M.V.; Gonzalez, M.; Poueymirou, W.T.; Kline, W.O.; Na, E.; Zlotchenko, E.; Stitt, T.N.; Economides, A.N.; Yancopoulos, G.D.; Glass, D.J. Conditional Activation of Akt in Adult Skeletal Muscle Induces Rapid Hypertrophy. Mol. Cell Biol. 2004, 24, 9295–9304. [Google Scholar]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541. [Google Scholar]
- Nader, G.A.; Esser, K.A. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J. Appl. Physiol. 2001, 90, 1936–1942. [Google Scholar]
- Coffey, V.G.; Zhong, Z.; Shield, A.; Canny, B.J.; Chibalin, A.V.; Zierath, J.R.; Hawley, J.A. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB 2006, 20, 190–192. [Google Scholar]
- Dreyer, H.C.; Fujita, S.; Cadenas, J.G.; Chinkes, D.L.; Volpi, E.; Rasmussen, B.B. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J. Physiol. 2006, 576, 613–624. [Google Scholar]
- Reynolds, T.H.; Bodine, S.C.; Lawrence, J.C. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J. Biol. Chem. 2002, 277, 17657–17662. [Google Scholar]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Jefferson, L.S. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine 1. J. Nutr. 2001, 131, 856–860. [Google Scholar]
- Brunn, G.J.; Williams, J.; Sabers, C.; Wiederrecht, G.; Lawrence, J.C., Jr; Abraham, R.T. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996, 15, 5256. [Google Scholar] [PubMed]
- Kimball, S.R.; Jefferson, L.S. Molecular mechanisms through which amino acids mediate signaling through the mammalian target of rapamycin. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 39. [Google Scholar]
- Mothe-Satney, I.; Gautier, N.; Hinault, C.; Lawrence, J.C, Jr; Van Obberghen, E. In rat hepatocytes glucagon increases mammalian target of rapamycin phosphorylation on serine 2448 but antagonizes the phosphorylation of its downstream targets induced by insulin and amino acids. J. Biol. Chem. 2004, 279, 42628. [Google Scholar] [PubMed]
- MacKenzie, M.G.; Hamilton, D.L.; Murray, J.T.; Taylor, P.M.; Baar, K. Vps34 is activated following high resistance contraction. J. Physiol. 2008, 587. [Google Scholar]
- Baar, K.; Esser, K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am. J. Physiol. Cell Physiol. 1999, 276, 120–127. [Google Scholar]
- Pearson, R.B.; Dennis, P.B.; Han, J.W.; Williamson, N.A.; Kozma, S.C.; Wettenhall, R.E.; Thomas, G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995, 14, 5279. [Google Scholar]
- Crozier, S.J.; Kimball, S.R.; Emmert, S.W.; Anthony, J.C.; Jefferson, L.S. Oral leucine administration stimulates protein synthesis in rat skeletal muscle 1. J. Nutr. 2005, 135, 376–382. [Google Scholar]
- Koopman, R.; Zorenc, A.H.G.; Gransier, R.J.J.; Cameron-Smith, D.; van Loon, L.J.C. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am. J. Physiol. Endocrinol. Metab. 2006, 290, 1245–1252. [Google Scholar]
- Long, W.; Saffer, L.; Wei, L.; Barrett, E.J. Amino acids regulate skeletal muscle PHAS-I and p70 S6-kinase phosphorylation independently of insulin. Am. J. Physiol. Endocrinol. Metab. 2000, 279, 301–306. [Google Scholar]
- Gingras, A.C.; Gygi, S.P.; Raught, B.; Polakiewicz, R.D.; Abraham, R.T.; Hoekstra, M.F.; Aebersold, R.; Sonenberg, N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999, 13, 1422–1437. [Google Scholar]
- Huth, P.J.; Miller, G.D.; Brown, P. Milk - A key to new products. Dairy Foods 2003, 46–49. [Google Scholar]
- Farnfield, M.M.; Trenerry, C.; Carey, K.A.; Cameron-Smith, D. Plasma amino acid response after ingestion of different whey protein fractions. Int. J. Nutr. Food Sci. 2008, 8, 1–8. [Google Scholar]
- Cribb, P.J.; Williams, A.D.; Stathis, C.G.; Carey, M.F.; Hayes, A. Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Med. Sci. Sports Exerc. 2007, 39, 298. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Rasmussen, C.; Lancaster, S.; Starks, M.; Smith, P.; Melton, C.; Greenwood, M.; Almada, A.; Kreider, R. Impact of differing protein sources and a creatine containing nutritional formula after 12 weeks of resistance training. Nutrition 2007, 23, 647–656. [Google Scholar]
- Willoughby, D.S.; Stout, J.R.; Wilborn, C.D. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 2007, 32, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.J. Protein hydrolysis of animal feeds for amino acid content. J. Agric. Food Chem. 1985, 33, 722–725. [Google Scholar]
Supplementary Files
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farnfield, M.M.; Carey, K.A.; Gran, P.; Trenerry, M.K.; Cameron-Smith, D. Whey Protein Ingestion Activates mTOR-dependent Signalling after Resistance Exercise in Young Men: A Double-Blinded Randomized Controlled Trial. Nutrients 2009, 1, 263-275. https://doi.org/10.3390/nu1020263
Farnfield MM, Carey KA, Gran P, Trenerry MK, Cameron-Smith D. Whey Protein Ingestion Activates mTOR-dependent Signalling after Resistance Exercise in Young Men: A Double-Blinded Randomized Controlled Trial. Nutrients. 2009; 1(2):263-275. https://doi.org/10.3390/nu1020263
Chicago/Turabian StyleFarnfield, Michelle M., Kate A. Carey, Petra Gran, Marissa K. Trenerry, and David Cameron-Smith. 2009. "Whey Protein Ingestion Activates mTOR-dependent Signalling after Resistance Exercise in Young Men: A Double-Blinded Randomized Controlled Trial" Nutrients 1, no. 2: 263-275. https://doi.org/10.3390/nu1020263
APA StyleFarnfield, M. M., Carey, K. A., Gran, P., Trenerry, M. K., & Cameron-Smith, D. (2009). Whey Protein Ingestion Activates mTOR-dependent Signalling after Resistance Exercise in Young Men: A Double-Blinded Randomized Controlled Trial. Nutrients, 1(2), 263-275. https://doi.org/10.3390/nu1020263