Ethanolic Extracts of Artemisia apiacea Hance Improved Atopic Dermatitis-Like Skin Lesions In Vivo and Suppressed TNF-Alpha/IFN-Gamma–Induced Proinflammatory Chemokine Production In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Culture of Keratinocytes
2.3. Preparation of Ethanolic Extract of Artemisia Apiacea Hance (EAH)
2.4. Chromatographic Conditions and Sample Preparation
2.5. MTT Assay
2.6. Cytokine and Chemokine Analysis
2.7. Western Blot
2.8. Immunofluorescence Assay
2.9. Animals
2.10. Induction of AD and Drug Treatment
2.11. Ear Thickness Measurements and Histopathological Analysis
2.12. Statistical Analysis
3. Results
3.1. Measurement of the Representative Component in EAH by HPLC Analysis
3.2. Effects of EAH on HaCaT Cell Viability
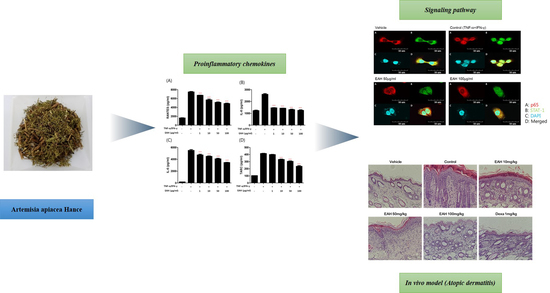
3.3. Effects of EAH on TNF-α/IFN-γ–Induced Production of Proinflammatory Cytokines and Chemokines in HaCaT Cells
3.4. Effects of EAH on Phosphorylation of Mitogen Activated Protein Kinases (MAPK), STAT-1, and NFκB-p65 in TNF-α/IFN-γ–Stimulated HaCaT Cells
3.5. Effects of EAH on NFκB-p65 and STAT-1 Translocation in TNF-α/IFN-γ-Stimulated HaCaT Cells
3.6. Effects of EAH on Development of DNCB-Induced AD Mouse Skin Lesions
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arellano, F.M.; Wentworth, C.E.; Arana, A.; Fernandez, C.; Paul, C.F. Risk of lymphoma following exposure to calcineurin inhibitors and topical steroids in patients with atopic dermatitis. J. Investig. Dermatol. 2007, 127, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Drucker, A.M.; Lebwohl, M.; Silverberg, J.I. A systematic review of the safety and efficacy of systemic corticosteroids in atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Bruin-Weller, M.; Flohr, C.; Ardern-Jones, M.R.; Barbarot, S.; Deleuran, M.; Bieber, T.; Vestergaard, C.; Brown, S.J.; Cork, M.J.; et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the international eczema council. J. Am. Acad. Dermatol. 2017, 77, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, I.S.; Ryu, J.S.; Yun, C.Y. House dust mite, dermatophagoides pteronissinus increases expression of MCP-1, IL-6, and IL-8 in human monocytic THP-1 cells. Cytokine 2008, 42, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Choi, B.M.; Jang, S.I. Sulforaphane suppresses TARC/CCL17 and MDC/CCL22 expression through heme oxygenase-1 and NF-kappaB in human keratinocytes. Arch. Pharm. Res. 2010, 33, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Roberts, M.H.; Yamamoto, N.; Sugiura, H.; Uehara, M.; Hopkin, J.M. Upregulating promoter polymorphisms of rantes relate to atopic dermatitis. Int. J. Immunogenet. 2006, 33, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Kim, M.S.; Le, M.Q.; Song, Y.S.; Bak, Y.; Ryu, H.W.; Oh, S.R.; Yoon, D.Y. Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and Nf-kB signaling. Phytomed. Int. J. Phytother. Phytopharmacol. 2017, 24, 96–103. [Google Scholar]
- Yang, J.H.; Hwang, Y.H.; Gu, M.J.; Cho, W.K.; Ma, J.Y. Ethanol extracts of Sanguisorba officinalis L. Suppress TNF-alpha/IFN-gamma-induced pro-inflammatory chemokine production in HaCaT cells. Phytomed. Int. J. Phytother. Phytopharmacol. 2015, 22, 1262–1268. [Google Scholar]
- Yang, H.J.; Kim, M.J.; Kang, S.; Moon, N.R.; Kim, D.S.; Lee, N.R.; Kim, K.S.; Park, S. Topical treatments of Saussurea costus root and Thuja orientalis L. Synergistically alleviate atopic dermatitis-like skin lesions by inhibiting protease-activated receptor-2 and NF-kappaB signaling in HaCaT cells and Nc/Nga mice. J. Ethnopharmacol. 2017, 199, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Weon, J.B.; Yun, B.R.; Eom, M.R.; Ma, C.J. Simultaneous determination three phytosterol compounds, campesterol, stigmasterol and daucosterol in Artemisia apiacea by high performance liquid chromatography-diode array ultraviolet/visible detector. Pharmacogn. Mag. 2015, 11, 297–303. [Google Scholar] [PubMed]
- Ryu, J.C.; Park, S.M.; Hwangbo, M.; Byun, S.H.; Ku, S.K.; Kim, Y.W.; Kim, S.C.; Jee, S.Y.; Cho, I.J. Methanol extract of Artemisia apiacea Hance attenuates the expression of inflammatory mediators via NF-kappaB inactivation. Evid. Based Complement. Altern. Med. 2013, 2013, 494681. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, Z.; Geng, Y. Anti-allergic effect of artemisia extract in rats. Exp. Ther. Med. 2016, 12, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.J.; Dang, H.T.; Han, S.C.; Kang, N.J.; Koo, D.H.; Koh, Y.S.; Hyun, J.W.; Kang, H.K.; Jung, J.H.; Yoo, E.S. Methyl 5-chloro-4,5-didehydrojasmonate (J7) inhibits macrophage-derived chemokine production via down-regulation of the signal transducers and activators of transcription 1 pathway in HaCaT human keratinocytes. Chem. Pharm. Bull. 2013, 61, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Yoo, J.M.; Cho, W.K.; Ma, J.Y. Ethanol extract of sanguisorbae radix inhibits mast cell degranulation and suppresses 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions. Mediat. Inflamm. 2016, 2016, 2947390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.W.; Ni, F.Y.; Song, Y.L.; Wang, S.Y.; Huang, W.Z.; Wang, Z.Z.; Xiao, W. Chemical constituents from Artemisia annua. Zhongguo Zhong Yao Za Zhi 2014, 39, 4816–4821. [Google Scholar] [PubMed]
- Sohn, E.H.; Jang, S.A.; Lee, C.H.; Jang, K.H.; Kang, S.C.; Park, H.J.; Pyo, S. Effects of korean red ginseng extract for the treatment of atopic dermatitis-like skin lesions in mice. J. Ginseng Res. 2011, 35, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, Y.J.; Lee, J.Y.; Park, Y.M. Topical application of eupatilin ameliorates atopic dermatitis-like skin lesions in Nc/Nga mice. Ann. Dermatol. 2017, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Ma, K.H.; Liu, P.S.; Chen, B.W.; Chueh, S.H. Baicalein increases keratin 1 and 10 expression in HaCaT keratinocytes via TRPV4 receptor activation. Exp. Dermatol. 2016, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lim, Y.Y.; Kim, H.M.; Park, Y.M.; Kang, H.; Kim, B.J. Synergistic inhibition of tumor necrosis factor-alpha-stimulated pro-inflammatory cytokine expression in HaCaT cells by a combination of rapamycin and mycophenolic acid. Ann. Dermatol. 2015, 27, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, Y.S.; Kim, H.K. Illicium verum extract inhibits TNF-alpha-and IFN-gamma-induced expression of chemokines and cytokines in human keratinocytes. J. Ethnopharmacol. 2012, 144, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Sokolowska-Wojdylo, M.; Ruckemann-Dziurdzinska, K.; Roszkiewicz, J.; Nowicki, R.J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatologii I Alergologii 2014, 31, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Siddiqi, M.H.; Aceituno, V.C.; Simu, S.Y.; Zhang, J.; Jimenez Perez, Z.E.; Kim, Y.J.; Yang, D.C. Ginsenoside Rg5:Rk1 attenuates TNF-alpha/IFN-gamma-induced production of thymus- and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-kappaB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Jin, S.E.; Kim, O.S.; Shin, H.K.; Jeong, S.J. Alantolactone from Saussurea lappa exerts antiinflammatory effects by inhibiting chemokine production and STAT1 phosphorylation in TNF-alpha and IFN-gamma-induced in HaCaT cells. Phytother. Res. 2015, 29, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Lim, H.S.; Seo, C.S.; Kim, J.H.; Jin, S.E.; Yoo, S.R.; Shin, H.K. Traditional herbal formula jakyakgamcho-tang (Paeonia lactiflora and Glycyrrhiza uralensis) impairs inflammatory chemokine production by inhibiting activation of STAT1 and NF-kappaB in HaCaT cells. Phytomed. Int. J. Phytother. Phytopharmacol. 2015, 22, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, M.S.; Jeong, G.S.; Yoon, J. Xanthii fructus extract inhibits TNF-alpha/IFN-gamma-induced Th2-chemokines production via blockade of NF-kappaB, STAT1 and p38-MAPK activation in human epidermal keratinocytes. J. Ethnopharmacol. 2015, 171, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Liu, J.; Wang, J.; Luo, Q.; Zhang, H.; Liu, B.; Xu, F.; Pang, Q.; Liu, Y.; Dong, J. Icariin inhibits TNF-alpha/IFN-gamma induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int. Immunopharmacol. 2015, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kim, S.H. Inhibitory effect of galangin on atopic dermatitis-like skin lesions. Food Chem. Toxicol. 2014, 68, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.J.; Han, S.C.; Yi, E.J.; Kang, H.K.; Yoo, E.S. The inhibitory effect of premature citrus unshiu extract on atopic dermatitis in vitro and in vivo. Toxicol. Res. 2011, 27, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ki, N.Y.; Park, E.J.; Sung, I.; Ju, S.A.; Kim, K.U.; Kim, M.R.; Song, D.Y.; Lee, M.J.; Kim, H.S.; Kang, B.H.; et al. The hot-water extract of smilacis chinae rhizome suppresses 2,4-dinitrochlorobenzene and house dust mite-induced atopic dermatitis-like skin lesions in mice. Phytother. Res. 2016, 30, 636–645. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-H.; Lee, E.; Lee, B.; Cho, W.-K.; Ma, J.Y.; Park, K.-I. Ethanolic Extracts of Artemisia apiacea Hance Improved Atopic Dermatitis-Like Skin Lesions In Vivo and Suppressed TNF-Alpha/IFN-Gamma–Induced Proinflammatory Chemokine Production In Vitro. Nutrients 2018, 10, 806. https://doi.org/10.3390/nu10070806
Yang J-H, Lee E, Lee B, Cho W-K, Ma JY, Park K-I. Ethanolic Extracts of Artemisia apiacea Hance Improved Atopic Dermatitis-Like Skin Lesions In Vivo and Suppressed TNF-Alpha/IFN-Gamma–Induced Proinflammatory Chemokine Production In Vitro. Nutrients. 2018; 10(7):806. https://doi.org/10.3390/nu10070806
Chicago/Turabian StyleYang, Ju-Hye, Esther Lee, BoHyoung Lee, Won-Kyung Cho, Jin Yeul Ma, and Kwang-Il Park. 2018. "Ethanolic Extracts of Artemisia apiacea Hance Improved Atopic Dermatitis-Like Skin Lesions In Vivo and Suppressed TNF-Alpha/IFN-Gamma–Induced Proinflammatory Chemokine Production In Vitro" Nutrients 10, no. 7: 806. https://doi.org/10.3390/nu10070806
APA StyleYang, J. -H., Lee, E., Lee, B., Cho, W. -K., Ma, J. Y., & Park, K. -I. (2018). Ethanolic Extracts of Artemisia apiacea Hance Improved Atopic Dermatitis-Like Skin Lesions In Vivo and Suppressed TNF-Alpha/IFN-Gamma–Induced Proinflammatory Chemokine Production In Vitro. Nutrients, 10(7), 806. https://doi.org/10.3390/nu10070806