Effect of Milk Fermented with Lactobacillus fermentum on the Inflammatory Response in Mice
Abstract
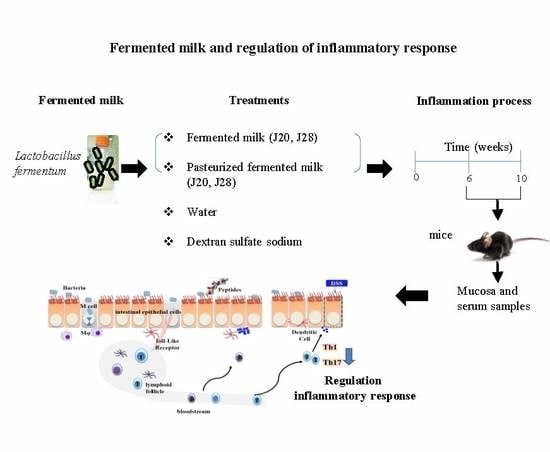
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Milk
2.2. Characterization of Fermented Milk
2.3. Determination of Exopolysaccharide
2.4. Animal Study
2.4.1. Serum, Organ, and Mucosa Collection
2.4.2. Quantification of Cytokines
2.4.3. Histological Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Anti-Inflammatory Response
Histological Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chami, B.; Yeung, A.W.S.; van Vreden, C.; King, N.J.C.; Bao, S. The role of CXCR3 in DSS-induced colitis. PLoS ONE 2014, 9, e101622. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Murray, P.J. Cytokine signaling modules in inflammatory responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Kita, M.; Shin-Ya, M.; Kishida, T.; Urano, A.; Takada, R.; Sakagami, J.; Imanishi, J.; Iwakura, Y.; Okanoue, T.; et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 2008, 377, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.T.; Elson, C.O.; Fouser, L.A.; Kolls, J.K. The Th17 Pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 477–512. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Qin, H.; Wang, L.; Benveniste, E.N.; Elson, C.O.; Cong, Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J. Immunol. 2011, 186, 6313–6318. [Google Scholar] [CrossRef] [PubMed]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.E.; Conklin, L.S.; Centola, M.; Li, X. Distinct cytokines patterns identified from multiplex profiles of murine DSS and TNBS-Induced Colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Aden, K.; Rehman, A.; Falk-Paulsen, M.; Secher, T.; Kuiper, J.; Tran, F.; Pfeuffer, S.; Sheibani-Tezerji, R.; Breuer, A.; Luzius, A.; et al. Epithelial IL-23R signaling licenses protective IL-22 responses in intestinal inflammation. Cell Rep. 2016, 16, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; van Bergenhenegouwen, J.; Overbeek, S.; van de Kant, H.J.G.; Garssen, J.; Folkerts, G.; Vos, P.; Morgan, M.E.; Kraneveld, A.D. Bifidobacterium breve attenuates murine dextran sodium sulfate-induced colitis and increases regulatory T cell responses. PLoS ONE 2014, 9, e95441. [Google Scholar] [CrossRef] [PubMed]
- Zakostelska, Z.; Kverka, M.; Klimesova, K.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Hornova, M.; Srutkova, D.; Hudcovic, T.; Ridl, J.; et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE 2011, 6, e27961. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zheng, C.Q.; Meng, F.J.; Zhou, Z.; Sang, L.X.; Jiang, M. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol. Cell. Biochem. 2013, 374, 1–11. [Google Scholar] [PubMed]
- Chiba, Y.; Shida, K.; Nagata, S.; Wada, M.; Bian, L.; Wang, C.; Shimizu, T.; Yamashiro, Y.; Kiyoshima-Shibata, J.; Nanno, M.; et al. Well-controlled proinflammatory cytokine responses of Peyer’s patch cells to probiotic Lactobacillus casei. Immunology 2010, 130, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Herías, M.V.; Koninkx, J.F.; Vos, J.G.; Huis in’t Veld, J.H.; van Dijk, J.E. Probiotic effects of Lactobacillus casei on DSS-induced ulcerative colitis in mice. Int. J. Food Microbiol. 2005, 103, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, A.; Umesaki, Y. Rationale for Using of Bifidobacterium probiotic strains-fermented milk against colitis based on animal experiments and clinical trials. Probiotics Antimicrob. Proteins 2008, 1, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.R.; Shandilya, U.K.; Kansal, V.K. Immunoprotective effect of probiotic Dahi Containing Lactobacillus acidophilus and Bifidobacterium bifidum on dextran sodium sulfate-induced ulcerative colitis in mice. Probiotics Antimicrob. Proteins 2012, 4, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Yoda, K.; Miyazawa, K.; Hosoda, M.; Hiramatsu, M.; Yan, F.; He, F. Lactobacillus GG-fermented milk prevents DSS-induced colitis and regulates intestinal epithelial homeostasis through activation of epidermal growth factor receptor. Eur. J. Nutr. 2014, 53, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Tallon, R.; Bressollier, P.; Urdaci, M.C. Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 2003, 154, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Nanda-Kumar, N.S.; Balamurugan, R.; Jayakanthan, K.; Pulimood, A.; Pugazhendhi, S.; Ramakrishna, B.S. Probiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfate. J. Gastroenterol. Hepatol. 2008, 23, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Algieri, F.; Rodriguez-Nogales, A. Effect of a Ropy Bifidobacterium animalis subsp. lactis Strain orally administered on DSS-induced colitis mice Model. Front. Microbiol. 2016, 7, 868. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Song, M.; Li, J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am. J. Physiol. Regul. Intregr. Comp. Physiol. 2004, 286, 686–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degeest, B.; Janssens, B.; De Vuyst, L. Exopolysaccharide (EPS) biosynthesis by Lactobacillus sakei 0-1: Production kinetics, enzyme activities and EPS yields. J. Appl. Microbiol. 2001, 91, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.E.; Zheng, B.; Koelink, P.J.; van de Kant, H.J.G.; Haazen, L.C.; van Roest, M.; Garssen, J.; Folkerts, G.; Kraneveld, A.D. New Perspective on Dextran Sodium sulfate colitis: Antigen-specific T cell development during intestinal inflammation. PLoS ONE 2013, 8, e69936. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M. The spleen in local and systemic regulation of immunity. Immunity 2014, 39, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Sanderson, I. Dextran sulfate Sodium-Induced inflammation is enhanced by intestinal epithelial cell chemokine expression in mice. Pediatr. Res. 2003, 53, 143–147. [Google Scholar] [PubMed]
- Fischer, A. Human immunodeficiency: Connecting STATA3, Th17 and human mucosal immunity. Immunol. Cell Biol. 2008, 86, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, L.; Lundy, S.; Sarkar, S.; Fox, D. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Crome, S.Q.; Wang, A.Y.; Levings, M.K. Translational mini-review series on Th17 cells: Function and regulation of human T helper 17 cells in health and disease. Clin. Exp. Immunol. 2010, 159, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Andoh, A.; Araki, Y.; Bamba, T.; Fujiyama, Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 2004, 110, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Yadav, P.K.; Su, J.L.; Wang, J.S.; Fei, K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 5784–5788. [Google Scholar] [CrossRef] [PubMed]
- Granier, A.; Goulet, O.; Hoarau, C. Fermentation products: Immunological effects on human and animal models. Pediatr. Res. 2013, 74, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; Nunes, C.; Pinto, P.; Re, R.; Rémond, D.; et al. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 2017, 57, 2497–2525. [Google Scholar] [CrossRef] [PubMed]
- Owaga, E.; Hsieh, R.-H.; Mugendi, B.; Masuku, S.; Shinh, C.-K.; Chang, J.-S. Th17 cells as potential probiotic therapeutic targets in inflammatory bowel diseases. Int. J. Mol. Sci. 2015, 16, 20841–20858. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Han, S.Y.; Bae, E.A.; Huh, C.H.S.; Ahn, Y.T.; Lee, J.H.K.; Kim, D.H. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. Int. Immunopharmacol. 2008, 8, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Håversen, L.; Ohlsson, B.G.; Hahn-Zoric, M.; Hanson, L.Å.; Mattsby-Baltzer, I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-κB. Cell. Immunol. 2002, 220, 83–95. [Google Scholar] [CrossRef]
- Matsumoto, S.; Watanabe, N.; Imaka, A.; Okabe, Y. Preventive effects of Bifidobacterium and Lactobacillus-fermented milk on the development of inflammatory bowel disease in senescence-accelerated mouse P1/Yit strain mice. Digestion 2001, 64, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Haileselassie, Y.; Navis, M.; Vu, N.; Qazi, K.R.; Rethi, B.; Sverremark-Ekström, E. Postbiotic modulation of retinoic acid imprinted mucosal-like dendritic cells by probiotic Lactobacillus reuteri 17938 in vitro. Front. Immunol. 2016, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Mirpuri, J.; Sotnikov, I.; Denning, T.L.; Yarovinsky, F.; Parkos, C.A.; Denning, P.W.; Louis, N.A. Lactobacillus rhamnosus (LGG) regulates IL-10 signaling in the developing murine colon through upregulation of the IL-10R2 receptor subunit. PLoS ONE 2012, 7, e51955. [Google Scholar] [CrossRef] [PubMed]
- Krieglstein, C.F.; Cerwinka, W.H.; Laroux, F.S.; Salter, J.W.; Russell, J.M.; Schuermann, G.; Grisham, M.B.; Ross, C.R.; Granger, D.N. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: Divergent roles of superoxide and nitric oxide. J. Exp. Med. 2001, 194, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Menchen, L.; Colon, A.L.; Madrigal, J.L.; Beltran, L.; Botella, S.; Lizasoain, I.; Leza, J.C.; Moro, M.A.; Menchen, P.; Cos, E.; et al. Activity of inducible and neuronal nitric oxide synthases in colonic mucosa predicts progression of ulcerative colitis. Am. J. Gastroenterol. 2004, 99, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nishio, H.; Tanigawa, T.; Yamagami, H.; Okazaki, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G506–G513. [Google Scholar] [CrossRef] [PubMed]





| Fermented Milk | Cell Concentration (Log CFU/mL) | Lactic Acid (%) | Exopolysaccharides (mg/mL) | Total Protein (%) |
|---|---|---|---|---|
| FM-J20 | 9.0 ± 0.01 a | 0.72 ± 0.01 a | 50.99 ± 0.10 a | 2.80 ± 0.04 a |
| PFM-J20 | ND | 0.75 ± 0.03 a | 61.00 ± 0.15 a | 2.71 ± 0.09 a |
| FM-J28 | 9.04 ± 0.01 a | 0.90 ± 0.01 a | 79.59 ± 0.05 b | 3.01 ± 0.05 a |
| PFM-J28 | ND | 0.87±0.01 a | 77.30 ± 0.01 b | 2.88 ± 0.07 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-López, L.; Hernández-Mendoza, A.; Mata-Haro, V.; Vallejo-Córdoba, B.; Wall-Medrano, A.; Astiazarán-García, H.; Estrada-Montoya, M.D.C.; González-Córdova, A.F. Effect of Milk Fermented with Lactobacillus fermentum on the Inflammatory Response in Mice. Nutrients 2018, 10, 1039. https://doi.org/10.3390/nu10081039
Santiago-López L, Hernández-Mendoza A, Mata-Haro V, Vallejo-Córdoba B, Wall-Medrano A, Astiazarán-García H, Estrada-Montoya MDC, González-Córdova AF. Effect of Milk Fermented with Lactobacillus fermentum on the Inflammatory Response in Mice. Nutrients. 2018; 10(8):1039. https://doi.org/10.3390/nu10081039
Chicago/Turabian StyleSantiago-López, Lourdes, Adrián Hernández-Mendoza, Verónica Mata-Haro, Belinda Vallejo-Córdoba, Abraham Wall-Medrano, Humberto Astiazarán-García, María Del Carmen Estrada-Montoya, and Aarón F. González-Córdova. 2018. "Effect of Milk Fermented with Lactobacillus fermentum on the Inflammatory Response in Mice" Nutrients 10, no. 8: 1039. https://doi.org/10.3390/nu10081039
APA StyleSantiago-López, L., Hernández-Mendoza, A., Mata-Haro, V., Vallejo-Córdoba, B., Wall-Medrano, A., Astiazarán-García, H., Estrada-Montoya, M. D. C., & González-Córdova, A. F. (2018). Effect of Milk Fermented with Lactobacillus fermentum on the Inflammatory Response in Mice. Nutrients, 10(8), 1039. https://doi.org/10.3390/nu10081039