1. Introduction
In recent years, the rapid Westernization of eating habits in Japan has led to an increased risk of obesity and other lifestyle diseases [
1]. Imbalance in various nutrients is a characteristic of the contemporary diet [
2]. Consuming a balance of these nutrients is thus considered important for the prevention of lifestyle diseases. Recently,
Euglena gracilis (Euglena) has gained attention as a new health food, as it is rich in vitamins, minerals, and other nutrients [
3], and also contains the insoluble fiber paramylon as a characteristic component. Paramylon, a triple-helical, high-polymer substance composed of linear β-1,3-glucans, is known to prevent obesity and diabetes [
4,
5]. Insoluble fiber absorbs water easily, increasing stool quantities and easing bowel movements. It also functions to prevent colorectal cancer by eliminating harmful substances from the body [
6]. Insoluble fiber includes celluloses, hemicelluloses, and lignins, whereas soluble fiber comprises pectins, gums, and reserved polysaccharides [
7,
8]. Soluble fiber inhibits the absorption of glucose and fats from the intestinal tract and functions to lower blood sugar and lipid levels [
9]. Through fermentation by intestinal microbiota, soluble fiber also yields the production of short-chain fatty acids, which are used by large intestinal mucosal cells as an energy source to enhance their protective effects on the intestine [
10]. Paramylon and other fiber types contained in Euglena are almost all insoluble.
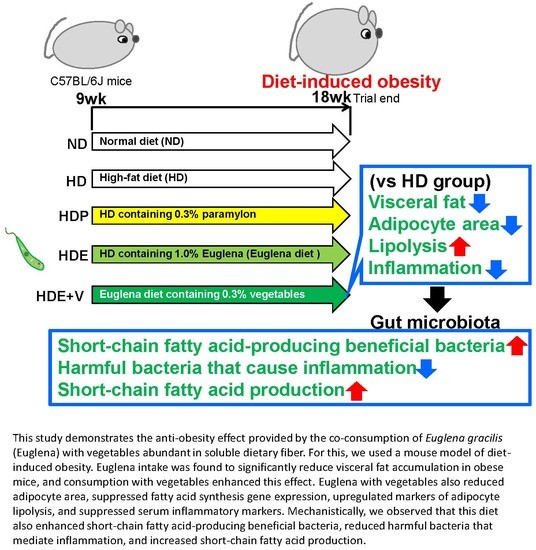
Many studies have been performed on Euglena and paramylon, but few studies have explored ways to increase their efficacy. Accordingly, we hypothesized that a balanced fiber intake, focusing on vegetables that are rich in soluble fiber, could enhance the efficacy of Euglena. Soluble fiber in vegetables, such as barley leaf, kale, and ashitaba, has been found to delay excess nutrient absorption in the intestine and promote short-chain fatty acid production through intestinal fermentation, thereby improving the intestinal environment [
11]. Our previous study showed that Euglena intake could significantly decrease visceral fat in normal mice, which was further augmented by simultaneous vegetable consumption [
12]. Further, we reported that the simultaneous consumption of Euglena and vegetables decreased adipocyte area, suppressed gene expression related to fatty acid synthesis, increased gene expression related to adipocyte proliferation and lipolysis, and suppressed serum inflammation parameters. Mechanistically, Euglena increased short chain-fatty acid-producing beneficial bacteria and decreased harmful bacteria that cause inflammation in the intestinal microbiota and enhanced short-chain fatty acid production. As the simultaneous consumption of Euglena and vegetables yielded beneficial effects in normal mice, we expected that this would also have an anti-obesity effect. Thus, in this study, we examined the effect of Euglena and vegetables using a mouse model of high-fat diet-induced obesity.
4. Discussion
In this study, for which the objective was to increase the nutritional value of insoluble fiber-rich Euglena, we determined whether the consumption of soluble fiber-rich vegetables could augment its anti-obesity effect. Results showed that this was the case, with the consumption of paramylon and Euglena resulting in a significant decrease of visceral fat in obese mice. Histological observation of white adipose tissue and the analysis of gene expression related to lipid metabolism showed that the simultaneous consumption of Euglena and vegetables decreased adipocyte area, suppressed gene expression related to fatty acid synthesis, and increased gene expression related to adipocyte lipolysis. We also observed a decrease in serum inflammation parameters. To explore the associated mechanisms, we examined the effect of Euglena on the intestinal microbiota, and observed the following: (1) Increases in beneficial bacteria (which were decreased due to obesity) that produce short-chain fatty acids and in beneficial bacteria that promote vitamin synthesis; (2) a decrease in harmful bacteria that cause inflammation, which had increased due to obesity; (3) an increase in short-chain fatty acids levels. Therefore, we confirmed that the simultaneous consumption of vegetables enhanced the anti-inflammatory effect of Euglena and its ability to suppress visceral fat accumulation; results suggested that changes in intestinal microbiota were involved in this effect.
The final body weight of obese mice increased significantly due to high fat intake, but paramylon or Euglena intake did not affect this. Moreover, due to the high-fat diet intake, the amount of feed administered was significantly decreased, but there was no difference in caloric intake between the groups. Thus, these changes likely originated from the high-fat diet. However, there was a decrease in the weight of white adipose tissue due to paramylon and Euglena intake, and in the case of perinephric and epididymal adipose tissue, this change was particularly pronounced. In studies using rat models of type 2 diabetes, visceral fat accumulation was inhibited by a diet including paramylon and Euglena, with the latter being more effective [
3,
5]. This difference was believed to be due to various beneficial components in Euglena in addition to paramylon. The same effect was observed in this study, and the effect of Euglena was further enhanced by vegetables. We previously demonstrated that one of the reasons why the traditional Japanese diet is more beneficial for preserving health than the contemporary diet is that it is important to consume a variety of food components [
14,
29,
30,
31]. Therefore, consuming small amounts of a variety of food components is extremely effective for preserving health, which suggests that the joint effect of Euglena and vegetables increased due to the diversity of food components.
Histological observation of epididymal adipose tissue showed that the adipocyte area exhibited a similar trend to visceral fat amounts. In addition, the expression of
Fasn,
Me, and
Srebp-1c [
32], which encode molecules that promote fat accumulation, exhibited the same trend as visceral fat levels. Meanwhile, the expression of
Ppar γ [
33], which promotes adipocyte proliferation and induces adipocyte miniaturization, and
Hsl [
34], which induces lipolysis and adipocyte miniaturization, exhibited the opposite trend. These findings demonstrated that the combined use of vegetables enhanced the effects of Euglena.
In this study, serum levels of IL-1β and IL-6 [
35], which are parameters of inflammation, decreased with Euglena consumption and decreased further when vegetables were added. This demonstrated that vegetables enhanced the anti-inflammatory effects of Euglena [
3]. Since maintaining low levels of inflammatory markers prevents the onset of aging-associated diseases and is thus useful for preserving health [
35], this suggests that the joint consumption of Euglena and vegetables could be beneficial for health and longevity. This potential was further suggested by the fact that the consumption of both Euglena and vegetables led to a significant decrease in the amount of over-oxidized fat and a reduction in oxidative stress.
To examine the mechanism associated with the anti-obesity and anti-inflammatory effects of Euglena, we investigated its effect on intestinal microbiota. Genera that increased with the consumption of a high-fat diet included
Sporosarcina,
Bacteria;Other;Other;Other;Other;Other,
Lactococcus,
Anaerotruncus,
Clostridiaceae;Other, and
Coprobacillus. In this study,
Sporosarcina,
Bacteria;Other;Other;Other;Other;Other,
Lactococcus, and
Clostridiaceae;Other increased with a high-fat diet, but decreased with paramylon consumption, decreased further with Euglena consumption, and decreased even further with the consumption of both Euglena and vegetables. This is a similar tendency to that observed for visceral fat. Levels of
Sporosarcina were previously found to increase when hepatitis was induced by artificial sweeteners [
36]. Therefore, increases in these genera might promote inflammation. Lactococcus includes useful bacteria that produce lactic acid but also putrefying bacteria [
37]. The function of
Bacteria;Other;Other;Other;Other;Other has not yet been determined, whereas
Clostridiaceae;Other includes harmful bacteria that cause food poisoning [
38]. Here, harmful bacteria such as those increased with obesity were inhibited with the consumption of paramylon, Euglena, and vegetables. In addition,
Coprobacillus functions to inhibit the proliferation of the toxin-producing bacterium
Clostridium difficile [
39], although we did not observe any major changes in this species due to paramylon or Euglena consumption.
Anaerotruncus produces short-chain fatty acids such as butyric acid and functions to inhibit inflammation [
40], and this experiment confirmed that paramylon and Euglena consumption could increase this genus.
Genera that decreased greatly with the consumption of a high-fat diet included
Erysipelotrichaceae;Other,
Bifidobacterium,
Lactobacillus,
F16;g__,
Clostridia;Other;Other;Other,
Enterococcaceae;Other, and
Escherichia. In this study,
Erysipelotrichaceae;Other,
Bifidobacterium,
Lactobacillus,
F16;g__, and
Clostridia;Other;Other;Other decreased with the high-fat diet, increased with paramylon consumption, increased further with Euglena consumption, and increased even further with the consumption of both Euglena and vegetables. This represented a trend to that observed for visceral fat amounts.
Erysipelotrichaceae;Other has been reported to increase with β-glucan and inulin consumption and is involved in the production of short-chain fatty acids [
41]. In this study, this group increased with the consumption of soluble fiber-rich vegetables, as did the short-chain fatty acid levels. Short-chain fatty acids exert an anti-inflammatory effect by strengthening the intestinal barrier function and an anti-obesity effect by increasing energy expenditure in the liver [
41,
42]. Consequently, this suggests that the effect of vegetables was due to increasing levels of short-chain fatty acids.
Bifidobacterium metabolizes sugars in the intestine to produce acetic acid and lactic acid [
43]. Acetic acid, which is a short-chain fatty acid, was also found to increase with the consumption of both Euglena and vegetables.
Lactobacillus metabolizes sugars in the intestine to produce lactic acid and encourages the production of GABA and other useful substances [
44,
45]. Lactic acid inhibits the growth of harmful bacteria that cannot survive in acidic conditions and exerts an anti-inflammatory effect. In this study, we observed that the consumption of Euglena and vegetables resulted in an increase in GABA, which possesses stress-reducing properties [
27,
45]. The function of
F16;g__ has not yet been determined.
Clostridia;Other;Other;Other includes harmful bacteria such as
Clostridrium perfringes [
46] and
Clostridrium difficile [
47], which cause contagious diseases. We did not find any remarkable changes in these species based on the consumption of paramylon or Euglena. In our experiments, obesity suppressed levels of beneficial bacteria, whereas the consumption of paramylon, Euglena, and vegetables promoted their abundance. In addition,
Enterococcaceae;Other, which produces lactic acid [
48], was scarcely detected in the high-fat diet groups of this study.
Escherichia encompasses many bacteria including
Escherichia coli.
E. coli is decreased in individuals who consume plenty of fiber [
49]. In this study, this species decreased with the consumption of paramylon and Euglena. Based on these data, we showed that the joint consumption of Euglena and vegetables resulted in an increase in beneficial bacteria and a decrease in harmful bacteria, which we believe was one of the mechanisms associated with the beneficial effects of vegetables.