A Newly Integrated Model for Intestinal Cholesterol Absorption and Efflux Reappraises How Plant Sterol Intake Reduces Circulating Cholesterol Levels
Abstract
:1. Introduction
2. Intestinal Cholesterol Absorption
2.1. An Overview of the Cholesterol Absorption Process
2.2. Passive Diffusion Mediates Intestinal Cholesterol Uptake
2.3. NPC1L1 Is a Major Gatekeeper for Cholesterol Assimilation in Enterocytes
3. The Small Intestine Excretes Endogenous Cholesterol
3.1. Trans-Intestinal Cholesterol Efflux
3.2. ATP-Binding Cassette G5/G8 Heterodimer Plays a Major Role in TICE
3.3. Cholesterol Absorption Inhibition by Liver X Receptor Agonism also Stimulates TICE
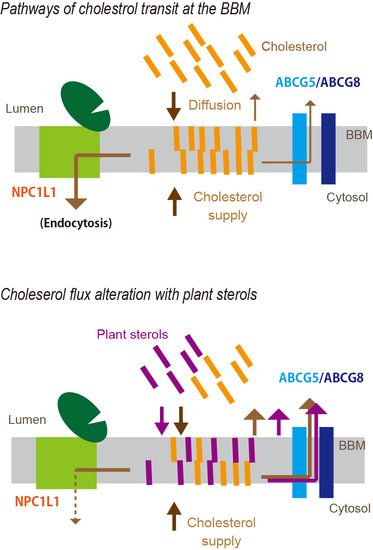
4. A Newly Integrated Model for Cholesterol Bidirectional Fluxes in the Small Intestine
5. Plant Sterols Modulate Cholesterol Flux in the BBM
5.1. Plant Sterols and Their LDL-C Lowering Effect
5.2. PSs as Modifiers of Cholesterol Flux in the Mucosa
5.3. PS Transition to the BBM
5.4. Possible Sites Where PSs Compete with the Absorption Process of Cholesterol
5.5. The Micellar Solubilization Hypothesis for PS-Mediated Cholesterol Absorption Inhibition
5.6. Association of PS Intake with LXR Activation
6. Trans-Intestinal Sterol Efflux
6.1. TISE
6.2. Association of Circulating PS Levels with the Newly Integrated Model for Intestinal Sterol Absorption and Efflux
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desmond, E.; Gribaldo, S. Phylogenomics of sterol synthesis: Insights into the origin, evolution, and diversity of a key eukaryotic feature. Genome Biol. Evol. 2009, 1, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Robinson, J.G.; Kowalewski, M.; Kołodziejczak, M.; Andreotti, F.; Bliden, K.; Tantry, U.; Kubica, J.; Raggi, P.; Gurbel, P.A. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: A systematic review and meta-analysis. JAMA 2018, 319, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). World Health Organization 2018. Available online: http://www.who.int/cardiovascular_diseases/en/ (accessed on 14 September 2018).
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Lin, X.; Racette, S.B.; Ma, L.; Wallendorf, M.; Davila-Roman, V.G.; Ostlund, R.E., Jr. Endogenous cholesterol excretion is negatively associated with carotid intima-media thickness in humans. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Nijstad, N.; Gautier, T.; Briand, F.; Rader, D.J.; Tietge, U.J.F. Biliary sterol secretion is required for functional in vivo reverse cholesterol transport in mice. Gastroenterology 2011, 140, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, W.J.; Hofmann, A.F.; Theodor, E. Absorption of cholesterol from a micellar solution: Intestinal perfusion studies in man. J. Clin. Investig. 1967, 46, 874–890. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, A.E.; Brufau, G.; Groen, A.K. Transintestinal cholesterol efflux. Curr. Opin. Lipidol. 2010, 21, 167–171. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, A.E.; Vrins, C.L.; van den Oever, K.; Kunne, C.; Oude Elferink, R.P.; Kuipers, F.; Groen, A.K. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology 2007, 133, 967–975. [Google Scholar] [CrossRef]
- Jakulj, L.; van Dijk, T.H.; de Boer, J.F.; Kootte, R.S.; Schonewille, M.; Paalvast, Y.; Boer, T.; Bloks, V.W.; Boverhof, R.; Nieuwdorp, M.; et al. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 2016, 24, 783–794. [Google Scholar] [CrossRef]
- de Boer, J.F.; Schonewille, M.; Boesjes, M.; Wolters, H.; Bloks, V.W.; Bos, T.; van Dijk, T.H.; Jurdzinski, A.; Boverhof, R.; Wolters, J.C.; et al. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology 2017, 152, 1126–1138. [Google Scholar] [CrossRef]
- Jakulj, L.; Vissers, M.N.; van Roomen, C.P.; van der Veen, J.N.; Vrins, C.L.J.; Kunne, C.; Stellaard, F.; Kastelein, J.J.P.; Groen, A.K. Ezetimibe stimulates faecal neutral sterol excretion depending on abcg8 function in mice. FEBS Lett. 2010, 584, 3625–3628. [Google Scholar] [CrossRef] [PubMed]
- Reeskamp, L.F.; Meessen, E.C.E.; Groen, A.K. Transintestinal cholesterol excretion in humans. Curr. Opin. Lipidol. 2018, 29, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Rudel, L.L. Intestinal cholesterol absorption. Curr. Opin. Lipidol. 1999, 10, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q. New concepts of mechanisms of intestinal cholesterol absorption. Ann. Hepatol. 2003, 2, 113–121. [Google Scholar] [PubMed]
- Brown, J.M.; Yu, L. Opposing gatekeepers of apical sterol transport: Niemann-Pick C1-like 1 (NPC1L1) and ATP-binding cassette transporters G5 and G8 (ABCG5/ABCG8). Immunol. Endocr. Metab. Agents Med. Chem. 2009, 9, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, S.; Patel, S.B. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: A review. Xenobiotica 2008, 38, 1119–1139. [Google Scholar] [CrossRef]
- Afonso, M.S.; Machado, R.M.; Lavrador, M.; Quintao, E.C.R.; Moore, K.; Lottenberg, A. Molecular pathways underlying cholesterol homeostasis. Nutrients 2018, 10, 760. [Google Scholar] [CrossRef]
- Thomson, A.B.; Schoeller, C.; Keelan, M.; Smith, L.; Clandinin, M.T. Lipid absorption: Passing through the unstirred layers, brush-border membrane, and beyond. Can. J. Physiol. Pharmacol. 1993, 71, 531–555. [Google Scholar] [CrossRef]
- Magot, T.; Verneau, C.; Lutton, C.; Chevallier, F. Origin and fate of cholesterol in rat plasma lipoproteins in vivo. I. Qualitative analysis. Ann. Nutr. Metab. 1985, 29, 147–159. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kinch, L.N.; Borek, D.M.; Wang, J.; Wang, J.; Urbatsch, I.L.; Xie, X.-S.; Grishin, N.V.; Cohen, J.C.; Otwinowski, Z.; et al. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature 2016, 533, 561–564. [Google Scholar] [CrossRef]
- Skov, M.; Tønnesen, C.K.; Hansen, G.H.; Danielsen, E.M. Dietary cholesterol induces trafficking of intestinal Niemann-Pick type C1 like 1 from the brush border to endosomes. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 300, G33–G40. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhou, Z.-S.; Li, N.; Bian, Y.; Wang, Y.-J.; Wang, L.-J.; Li, B.-L.; Song, B.-L. Ezetimibe blocks the internalization of NPC1L1 and cholesterol in mouse small intestine. J. Lipid Res. 2012, 53, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Perrodin, M.; Serougne, C.; Lutton, C. In vivo cholesterol synthesis by the rat digestive tract. II. A study of turnover. Reprod. Nutr. Dev. 1985, 25, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Christon, R.; Meslin, J.C.; Thevenoux, J.; Linard, A.; Leger, C.L.; Delpal, S. Effects of a low dietary linoleic acid level on intestinal morphology and enterocyte brush border membrane lipid composition. Reprod. Nutr. Dev. 1991, 31, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, H.; Dietschy, J.M. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J. Clin. Investig. 1976, 58, 97–108. [Google Scholar] [CrossRef]
- Chijiiwa, K.; Linscheer, W.G. Distribution and monomer activity of cholesterol in micellar bile salt: Effect of cholesterol level. Am. J. Physiol. 1987, 252, G309–G314. [Google Scholar] [CrossRef]
- Sylvén, C.; Borgström, B. Absorption and lymphatic transport of cholesterol in the rat. J. Lipid Res. 1968, 9, 596–601. [Google Scholar]
- Fujikawa, M.; Nakao, K.; Shimizu, R.; Akamatsu, M. QSAR study on permeability of hydrophobic compounds with artificial membranes. Bioorg. Med. Chem. 2007, 15, 3756–3767. [Google Scholar] [CrossRef]
- Compassi, S.; Werder, M.; Weber, F.E.; Boffelli, D.; Hauser, H.; Schulthess, G. Comparison of cholesterol and sitosterol uptake in different brush border membrane models. Biochemistry 1997, 36, 6643–6652. [Google Scholar] [CrossRef]
- Hauser, H.; Howell, K.; Dawson, R.M.; Bowyer, D.E. Rabbit small intestinal brush border membrane preparation and lipid composition. Biochim. Biophys. Acta 1980, 602, 567–577. [Google Scholar] [CrossRef]
- Knöpfel, M.; Davies, J.P.; Duong, P.T.; Kværnø, L.; Carreira, E.M.; Phillips, M.C.; Ioannou, Y.A.; Hauser, H. Multiple plasma membrane receptors but not NPC1L1 mediate high-affinity, ezetimibe-sensitive cholesterol uptake into the intestinal brush border membrane. Biochim. Biophys. Acta (BBA) 2007, 1771, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.V.; Drover, V.A.; Knopfel, M.; Dhanasekaran, P.; Hauser, H.; Phillips, M.C. Influence of class B scavenger receptors on cholesterol flux across the brush border membrane and intestinal absorption. J. Lipid Res. 2009, 50, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Field, F.J.; Watt, K.; Mathur, S.N. Ezetimibe interferes with cholesterol trafficking from the plasma membrane to the endoplasmic reticulum in Caco-2 cells. J. Lipid Res. 2007, 48, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Altmann, S.W.; Davis, H.R., Jr.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.-H.; Cao, J.; Qu, Y.-X.; Li, B.-L.; Song, B.-L. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.A.; Pfeffer, S.R. Ezetimibe-sensitive cholesterol uptake by NPC1L1 protein does not require endocytosis. Mol. Biol. Cell 2016, 27, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Qi, W.; Wang, L.-J.; Miao, H.-H.; Qu, Y.-X.; Li, B.-L.; Song, B.-L. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc. Natl. Acad. Sci. USA 2011, 108, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Li, N.; Chen, Z.-J.; Li, B.-L.; Song, B.-L. The small GTPase cdc42 interacts with Niemann-Pick C1 Like 1 (NPC1L1) and controls its movement from endocytic recycling compartment to plasma membrane in a cholesterol dependent manner. J. Biol. Chem. 2011, 286, 35933–35942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Ge, L.; Qi, W.; Zhang, L.; Miao, H.-H.; Li, B.-L.; Yang, M.; Song, B.-L. The N-terminal domain of NPC1L1 protein binds cholesterol and plays essential roles in cholesterol uptake. J. Biol. Chem. 2011, 286, 25088–25097. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, A.; Sun, L.-P.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Direct binding of cholesterol to the purified membrane region of SCAP: Mechanism for a sterol-sensing domain. Mol. Cell 2004, 15, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kamishikiryo, J.; Haraguchi, M.; Nakashima, S.; Tasaka, Y.; Narahara, H.; Sugihara, N.; Nakamura, T.; Morita, T. N-terminal domain of the cholesterol transporter Niemann–Pick C1-Like 1 (NPC1L1) is essential for α-tocopherol transport. Biochem. Biophys. Res. Commun. 2017, 486, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Abuasal, B.; Sylvester, P.W.; Kaddoumi, A. Intestinal absorption of γ-tocotrienol is mediated by Niemann-Pick C1-like 1: In situ rat intestinal perfusion studies. Drug Metab. Dispos. 2010, 38, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Narushima, K.; Takada, T.; Yamanashi, Y.; Suzuki, H. Niemann-Pick C1-like 1 mediates α-tocopherol transport. Mol. Pharmacol. 2008, 74, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Yamanashi, Y.; Konishi, K.; Yamamoto, T.; Toyoda, Y.; Masuo, Y.; Yamamoto, H.; Suzuki, H. NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci. Transl. Med. 2015, 7, 275ra23. [Google Scholar] [CrossRef]
- Reboul, E.; Borel, P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 2011, 50, 388–402. [Google Scholar] [CrossRef]
- Wilson, M.D.; Rudel, L.L. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J. Lipid Res. 1994, 35, 943–955. [Google Scholar]
- Dubois, C.; Armand, M.; Ferezou, J.; Beaumier, G.; Portugal, H.; Pauli, A.; Bernard, P.; Becue, T.; Lafont, H.; Lairon, D. Postprandial appearance of dietary deuterated cholesterol in the chylomicron fraction and whole plasma in healthy subjects. Am. J. Clin. Nutr. 1996, 64, 47–52. [Google Scholar] [CrossRef]
- Samuel, P.; Crouse, J.R.; Ahrens, E.H. Evaluation of an isotope ratio method for measurement of cholesterol absorption in man. J. Lipid Res. 1978, 19, 82–93. [Google Scholar]
- Cheng, S.H.; Stanley, M.M. Secretion of cholesterol by intestinal mucosa in patients with complete common bile duct obstruction. Proc. Soc. Exp. Biol. Med. 1959, 101, 223–225. [Google Scholar] [CrossRef]
- Moreau, F.; Blanchard, C.; Perret, C.; Flet, L.; Douane, F.; Frampas, E.; Mirallie, E.; Croyal, M.; Aguesse, A.; Krempf, M.; et al. In vivo evidence for transintestinal cholesterol efflux in patients with complete common bile duct obstruction. J. Clin. Lipidol. 2018. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Pijut, S.S.; Li, J.; Horn, J.; Bradford, E.M.; Leggas, M.; Barrett, T.A.; Graf, G.A. The combination of ezetimibe and ursodiol promotes fecal sterol excretion and reveals a G5G8-independent pathway for cholesterol elimination. J. Lipid Res. 2015, 56, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ma, Y.; Jia, L.; Ioannou, Y.A.; Davies, J.P.; Yu, L. Genetic inactivation of NPC1L1 protects against sitosterolemia in mice lacking ABCG5/ABCG8. J. Lipid Res. 2009, 50, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Inoue, I.; Takenaka, Y.; Ono, H.; Katayama, S.; Awata, T.; Murakoshi, T. Ezetimibe promotes brush border membrane-to-lumen cholesterol efflux in the small intestine. PLoS ONE 2016, 11, e0152207. [Google Scholar] [CrossRef] [PubMed]
- Briand, F.; Naik, S.U.; Fuki, I.; Millar, J.S.; Macphee, C.; Walker, M.; Billheimer, J.; Rothblat, G.; Rader, D.J. Both the peroxisome proliferator-activated receptor delta agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of HDL-derived cholesterol. Clin. Transl. Sci. 2009, 2, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Racette, S.B.; Ma, L.; Wallendorf, M.; Ostlund, R.E. Ezetimibe increases endogenous cholesterol excretion in humans. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.K.; Connor, W.E.; Lin, D.S.; McMurry, M.M.; Shulman, R.S. Sluggish sitosterol turnover and hepatic failure to excrete sitosterol into bile cause expansion of body pool of sitosterol in patients with sitosterolemia and xanthomatosis. Arterioscler. Thromb. Vasc. Biol. 1991, 11, 1287–1294. [Google Scholar] [CrossRef]
- Yu, L.; Li-Hawkins, J.; Hammer, R.E.; Berge, K.E.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Investig. 2002, 110, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Le May, C.; Berger, J.M.; Lespine, A.; Pillot, B.; Prieur, X.; Letessier, E.; Hussain, M.M.; Collet, X.; Cariou, B.; Costet, P. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Rose-Sperling, D.; Hellmich, U.A. Diverse relations between ABC transporters and lipids: An overview. Biochim. Biophys. Acta (BBA)–Biomembr. 2017, 1859, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Bonamassa, B.; Moschetta, A. Atherosclerosis: Lessons from LXR and the intestine. Trends Endocrinol. Metab. 2013, 24, 120–128. [Google Scholar] [CrossRef]
- Duval, C.; Touche, V.; Tailleux, A.; Fruchart, J.-C.; Fievet, C.; Clavey, V.; Staels, B.; Lestavel, S. Niemann–Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem. Biophys. Res. Commun. 2006, 340, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; York, J.; von Bergmann, K.; Lutjohann, D.; Cohen, J.C.; Hobbs, H.H. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J. Biol. Chem. 2003, 278, 15565–15570. [Google Scholar] [CrossRef] [PubMed]
- Kruit, J.K.; Plösch, T.; Havinga, R.; Boverhof, R.; Groot, P.H.E.; Groen, A.K.; Kuipers, F. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology 2005, 128, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Brufau, G.; Kuipers, F.; Lin, Y.; Trautwein, E.A.; Groen, A.K. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS ONE 2011, 6, e21576. [Google Scholar] [CrossRef] [PubMed]
- Bura, K.S.; Lord, C.; Marshall, S.; McDaniel, A.; Thomas, G.; Warrier, M.; Zhang, J.; Davis, M.A.; Sawyer, J.K.; Shah, R.; et al. Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J. Lipid Res. 2013, 54, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kennedy, S.; Sidhu, R.; Luo, J.; Ory, D.S.; Davidson, N.O. Liver X receptor agonist modulation of cholesterol efflux in mice with intestine-specific deletion of microsomal triglyceride transfer protein. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.D.; Valasek, M.A.; Repa, J.J.; Dietschy, J.M. Multiple mechanisms limit the accumulation of unesterified cholesterol in the small intestine of mice deficient in both ACAT2 and ABCA1. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1012–G1022. [Google Scholar] [CrossRef]
- Sehayek, E.; Hazen, S.L. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1296–1297. [Google Scholar] [CrossRef]
- Valasek, M.A.; Repa, J.J.; Quan, G.; Dietschy, J.M.; Turley, S.D. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G813–G822. [Google Scholar] [CrossRef]
- Magot, T.; Champarnaud, G.; Anfreville, R.; Lutton, C.; Chevallier, F. Origin and fate of cholesterol in rat plasma lipoproteins in vivo. II. Modelling of cholesterol absorption and its release into plasma lipoproteins. Ann. Nutr. Metab. 1985, 29, 160–174. [Google Scholar] [CrossRef]
- Feingold, K.R.; Wiley, M.H.; Moser, A.H.; Lau, D.T.; Lear, S.R.; Siperstein, M.D. De novo sterologenesis in intact primates. J. Lab. Clin. Med. 1982, 100, 405–410. [Google Scholar]
- Perrodin, M.; Lutton, C. In vivo cholesterol synthesis by the rat digestive tract. I. A topological study. Reprod. Nutr. Dev. 1985, 25, 647–657. [Google Scholar] [CrossRef]
- Borgstrom, B.; Radner, S.; Werner, B. Lymphatic transport of cholesterol in the human being. Effect of dietary cholesterol. Scand. J. Clin. Lab. Investig. 1970, 26, 227–235. [Google Scholar] [CrossRef]
- Beaumier-Gallon, G.; Dubois, C.; Senft, M.; Vergnes, M.-F.; Pauli, A.-M.; Portugal, H.; Lairon, D. Dietary cholesterol is secreted in intestinally derived chylomicrons during several subsequent postprandial phases in healthy humans. Am. J. Clin. Nutr. 2001, 73, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Sudhop, T.; Lütjohann, D.; von Bergmann, K. Sterol transporters: Targets of natural sterols and new lipid lowering drugs. Pharmacol. Ther. 2005, 105, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Nakazawa, T.; Nakamura, A.; Honda, C.; Endo, K.; Tsukada, M. Study of thermodynamic parameters for solubilization of plant sterol and stanol in bile salt micelles. Chem. Phys. Lipids 2008, 154, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, S.; Andersson, H.; Mulligan, A.; Bhaniani, A.; Welch, A.; Bingham, S.; Khaw, K.T.; Andersson, S.; Ellegård, L. Food sources of plant sterols in the EPIC Norfolk population. Eur. J. Clin. Nutr. 2007, 62, 695. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; Vermeer, M.A.; Hiemstra, H.; Ras, R.T. LDL-cholesterol lowering of plant sterols and stanols—Which factors influence their efficacy? Nutrients 2018, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; van der Schouw, Y.T.; Trautwein, E.A.; Sioen, I.; Dalmeijer, G.W.; Zock, P.L.; Beulens, J.W. Intake of phytosterols from natural sources and risk of cardiovascular disease in the european prospective investigation into cancer and nutrition-the netherlands (EPIC-NL) population. Eur. J. Prev. Cardiol. 2015, 22, 1067–1075. [Google Scholar] [CrossRef]
- Peterson, D.W. Effect of soybean sterols in the diet on plasma and liver cholesterol in chicks. Proc. Soc. Exp. Biol. Med. 1951, 78, 143–147. [Google Scholar] [CrossRef]
- Smet, E.D.; Mensink, R.P.; Plat, J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol. Nutr. Food Res. 2012, 56, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Tanaka, K.; Sugano, M.; Vahouny, G.V.; Gallo, L.L. Inhibition of cholesterol absorption in rats by plant sterols. J. Lipid Res. 1988, 29, 1573–1582. [Google Scholar] [PubMed]
- The Myocardial Infarction Genetics Consortium Investigators. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N. Engl. J. Med. 2014, 371, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Engelking, L.J.; McFarlane, M.R.; Li, C.K.; Liang, G. Blockade of cholesterol absorption by ezetimibe reveals a complex homeostatic network in enterocytes. J. Lipid Res. 2012, 53, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Telford, D.E.; Sutherland, B.G.; Edwards, J.Y.; Andrews, J.D.; Barrett, P.H.R.; Huff, M.W. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J. Lipid Res. 2007, 48, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Zmyslowski, A.; Szterk, A. Current knowledge on the mechanism of atherosclerosis and pro-atherosclerotic properties of oxysterols. Lipids Health Dis. 2017, 16, 188. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.; Haskard, D.; Khamis, R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis—Novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 2018, 29, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Que, X.; Hung, M.Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Maatta, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef]
- Rudkowska, I.; AbuMweis, S.S.; Nicolle, C.; Jones, P.J. Cholesterol-lowering efficacy of plant sterols in low-fat yogurt consumed as a snack or with a meal. J. Am. Coll. Nutr. 2008, 27, 588–595. [Google Scholar] [CrossRef]
- Jakulj, L.; Trip, M.D.; Sudhop, T.; von Bergmann, K.; Kastelein, J.J.P.; Vissers, M.N. Inhibition of cholesterol absorption by the combination of dietary plant sterols and ezetimibe: Effects on plasma lipid levels. J. Lipid Res. 2005, 46, 2692–2698. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Sugano, M. Some aspects of mechanism of inhibition of cholesterol absorption by β-sitosterol. Biochim. Biophys. Acta 1983, 732, 651–658. [Google Scholar] [CrossRef]
- Igel, M.; Giesa, U.; Lütjohann, D.; von Bergmann, K. Comparison of the intestinal uptake of cholesterol, plant sterols, and stanols in mice. J. Lipid Res. 2003, 44, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Bosner, M.S.; Ostlund, R.E.; Osofisan, O.; Grosklos, J.; Fritschle, C.; Lange, L.G. Assessment of percent cholesterol absorption in humans with stable isotopes. J. Lipid Res. 1993, 34, 1047–1053. [Google Scholar] [PubMed]
- Ostlund, R.E.; McGill, J.B.; Zeng, C.-M.; Covey, D.F.; Stearns, J.; Stenson, W.F.; Spilburg, C.A. Gastrointestinal absorption and plasma kinetics of soy δ5-phytosterols and phytostanols in humans. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E911–E916. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Inoue, I.; Takenaka, Y.; Ikegami, Y.; Kotani, N.; Shimada, A.; Noda, M.; Murakoshi, T. Luminal plant sterol promotes brush border membrane-to-lumen cholesterol efflux in the small intestine. J. Clin. Biochem. Nutr. 2018, 63, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, F.; Zhang, D.-W.; Ma, Y.; Xu, F.; Belani, J.D.; Cohen, J.C.; Hobbs, H.H.; Xie, X.-S. Sterol transfer by ABCG5 and ABCG8: In vitro assay and reconstitution. J. Biol. Chem. 2006, 281, 27894–27904. [Google Scholar] [CrossRef]
- Vrins, C.; Vink, E.; Vandenberghe, K.E.; Frijters, R.; Seppen, J.; Groen, A.K. The sterol transporting heterodimer ABCG5/ABCG8 requires bile salts to mediate cholesterol efflux. FEBS Lett. 2007, 581, 4616–4620. [Google Scholar] [CrossRef]
- Johnson, B.J.H.; Lee, J.-Y.; Pickert, A.; Urbatsch, I.L. Bile acids stimulate ATP hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry 2010, 49, 3403–3411. [Google Scholar] [CrossRef]
- Plösch, T.; Kruit, J.K.; Bloks, V.W.; Huijkman, N.C.A.; Havinga, R.; Duchateau, G.S.M.J.E.; Lin, Y.; Kuipers, F. Reduction of cholesterol absorption by dietary plant sterols and stanols in mice is independent of the ABCG5/8 transporter. J. Nutr. 2006, 136, 2135–2140. [Google Scholar] [CrossRef]
- Slota, T.; Kozlov, N.A.; Ammon, H.V. Comparison of cholesterol and β-sitosterol: Effects on jejunal fluid secretion induced by oleate, and absorption from mixed micellar solutions. Gut 1983, 24, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W.; Hang, J.; Dussault, P.H.; Carr, T.P. Phytosterol ester constituents affect micellar cholesterol solubility in model bile. Lipids 2010, 45, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I. Factors affecting intestinal absorption of cholesterol and plant sterols and stanols. J. Oleo Sci. 2015, 64, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; van Onselen, E.N.; van Heugten, M.M.; Mensink, R.P. Effects on serum lipids, lipoproteins and fat soluble antioxidant concentrations of consumption frequency of margarines and shortenings enriched with plant stanol esters. Eur. J. Clin. Nutr. 2000, 54, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Matvienko, O.A.; Lewis, D.S.; Swanson, M.; Arndt, B.; Rainwater, D.L.; Stewart, J.; Alekel, D.L. A single daily dose of soybean phytosterols in ground beef decreases serum total cholesterol and LDL cholesterol in young, mildly hypercholesterolemic men. Am. J. Clin. Nutr. 2002, 76, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Nichols, J.A.; Mensink, R.P. Plant sterols and stanols: Effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 2005, 46, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Calpe-Berdiel, L.; Escolà-Gil, J.C.; Ribas, V.; Navarro-Sastre, A.; Garcés-Garcés, J.; Blanco-Vaca, F. Changes in intestinal and liver global gene expression in response to a phytosterol-enriched diet. Atherosclerosis 2005, 181, 75–85. [Google Scholar] [CrossRef]
- Mashnafi, S.; Plat, J.; Mensink, R.P.; Baumgartner, S. Non-cholesterol sterol concentrations as biomarkers for cholesterol absorption and synthesis in different metabolic disorders: A systematic review. Nutrients 2019, 11, 124. [Google Scholar] [CrossRef]
- Ravid, Z.; Bendayan, M.; Delvin, E.; Sane, A.T.; Elchebly, M.; Lafond, J.; Lambert, M.; Mailhot, G.; Levy, E. Modulation of intestinal cholesterol absorption by high glucose levels: Impact on cholesterol transporters, regulatory enzymes, and transcription factors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G873–G885. [Google Scholar] [CrossRef]
- Malhotra, P.; Boddy, C.S.; Soni, V.; Saksena, S.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. D-glucose modulates intestinal Niemann-Pick C1 Like 1 (NPC1L1) gene expression via transcriptional regulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 304, G203–G210. [Google Scholar] [CrossRef]
- Lally, S.; Owens, D.; Tomkin, G.H. Genes that affect cholesterol synthesis, cholesterol absorption, and chylomicron assembly: The relationship between the liver and intestine in control and streptozotosin diabetic rats. Metabolism 2007, 56, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Lally, S.; Tan, C.Y.; Owens, D.; Tomkin, G.H. Messenger RNA levels of genes involved in dysregulation of postprandial lipoproteins in type 2 diabetes: The role of Niemann-Pick C1-Like 1, ATP-binding cassette, transporters G5 and G8, and of microsomal triglyceride transfer protein. Diabetologia 2006, 49, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.; Hayashi, A.A.; Webb, J.; Adeli, K. Postprandial dyslipidemia in insulin resistance: Mechanisms and role of intestinal insulin sensitivity. Atheroscler. Suppl. 2008, 9, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bloks, V.W.; Bakker-van Waarde, W.M.; Verkade, H.J.; Kema, I.P.; Wolters, H.; Vink, E.; Groen, A.K.; Kuipers, F. Down-regulation of hepatic and intestinal Abcg5 and abcg8 expression associated with altered sterol fluxes in rats with streptozotocin-induced diabetes. Diabetologia 2004, 47, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.A.; Webb, J.; Choi, J.; Baker, C.; Lino, M.; Trigatti, B.; Trajcevski, K.E.; Hawke, T.J.; Adeli, K. Intestinal SR-BI is upregulated in insulin-resistant states and is associated with overproduction of intestinal apoB48-containing lipoproteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G326–G337. [Google Scholar] [CrossRef] [PubMed]
- Naples, M.; Baker, C.; Lino, M.; Iqbal, J.; Hussain, M.M.; Adeli, K. Ezetimibe ameliorates intestinal chylomicron overproduction and improves glucose tolerance in a diet-induced hamster model of insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1043–G1052. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Lalonde, G.; Delvin, E.; Elchebly, M.; Précourt, L.P.; Seidah, N.G.; Spahis, S.; Rabasa-Lhoret, R.; Ziv, E. Intestinal and hepatic cholesterol carriers in diabetic Psammomys Obesus. Endocrinology 2010, 151, 958–970. [Google Scholar] [CrossRef]
- Lally, S.; Owens, D.; Tomkin, G.H. The different effect of pioglitazone as compared to insulin on expression of hepatic and intestinal genes regulating post-prandial lipoproteins in diabetes. Atherosclerosis 2007, 193, 343–351. [Google Scholar] [CrossRef]
- Borthwick, F.; Mangat, R.; Warnakula, S.; Jacome-Sosa, M.; Vine, D.F.; Proctor, S.D. Simvastatin treatment upregulates intestinal lipid secretion pathways in a rodent model of the metabolic syndrome. Atherosclerosis 2014, 232, 141–148. [Google Scholar] [CrossRef]
- Scoggan, K.A.; Gruber, H.; Chen, Q.; Plouffe, L.J.; Lefebvre, J.M.; Wang, B.; Bertinato, J.; L’Abbé, M.R.; Hayward, S.; Ratnayake, W.M.N. Increased incorporation of dietary plant sterols and cholesterol correlates with decreased expression of hepatic and intestinal Abcg5 and Abcg8 in diabetic BB rats. J. Nutr. Biochem. 2009, 20, 177–186. [Google Scholar] [CrossRef]
- Matthan, N.R.; Pencina, M.; LaRocque, J.M.; Jacques, P.F.; D’Agostino, R.B.; Schaefer, E.J.; Lichtenstein, A.H. Alterations in cholesterol absorption/synthesis markers characterize Framingham offspring study participants with CHD. J. Lipid Res. 2009, 50, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Assmann, G.; Cullen, P.; Kannenberg, F.; Schulte, H. Relationship between phytosterol levels and components of the metabolic syndrome in the procam study. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, O.; Baber, R.; Teupser, D. Plant sterols in food: No consensus in guidelines. Biochem. Biophys. Res. Commun. 2014, 446, 811–813. [Google Scholar] [CrossRef] [PubMed]
- 1Gylling, H.; Hallikainen, M.; Raitakari, O.T.; Laakso, M.; Vartiainen, E.; Salo, P.; Korpelainen, V.; Sundvall, J.; Miettinen, T.A. Long-term consumption of plant stanol and sterol esters, vascular function and genetic regulation. Br. J. Nutr. 2008, 101, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Luister, A.; Schött, H.F.; Husche, C.; Schäfers, H.-J.; Böhm, M.; Plat, J.; Gräber, S.; Lütjohann, D.; Laufs, U.; Weingärtner, O. Increased plant sterol deposition in vascular tissue characterizes patients with severe aortic stenosis and concomitant coronary artery disease. Steroids 2015, 99, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Schonewille, M.; Freark de Boer, J.; Mele, L.; Wolters, H.; Bloks, V.W.; Wolters, J.C.; Kuivenhoven, J.A.; Tietge, U.J.F.; Brufau, G.; Groen, A.K. Statins increase hepatic cholesterol synthesis and stimulate fecal cholesterol elimination in mice. J. Lipid Res. 2016, 57, 1455–1464. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, T.; Inoue, I.; Murakoshi, T. A Newly Integrated Model for Intestinal Cholesterol Absorption and Efflux Reappraises How Plant Sterol Intake Reduces Circulating Cholesterol Levels. Nutrients 2019, 11, 310. https://doi.org/10.3390/nu11020310
Nakano T, Inoue I, Murakoshi T. A Newly Integrated Model for Intestinal Cholesterol Absorption and Efflux Reappraises How Plant Sterol Intake Reduces Circulating Cholesterol Levels. Nutrients. 2019; 11(2):310. https://doi.org/10.3390/nu11020310
Chicago/Turabian StyleNakano, Takanari, Ikuo Inoue, and Takayuki Murakoshi. 2019. "A Newly Integrated Model for Intestinal Cholesterol Absorption and Efflux Reappraises How Plant Sterol Intake Reduces Circulating Cholesterol Levels" Nutrients 11, no. 2: 310. https://doi.org/10.3390/nu11020310
APA StyleNakano, T., Inoue, I., & Murakoshi, T. (2019). A Newly Integrated Model for Intestinal Cholesterol Absorption and Efflux Reappraises How Plant Sterol Intake Reduces Circulating Cholesterol Levels. Nutrients, 11(2), 310. https://doi.org/10.3390/nu11020310