Effect of Sucrose Ingestion at the End of a Critical Window that Increases Hypertension Susceptibility on Peripheral Mechanisms Regulating Blood Pressure in Rats. Role of Sirtuins 1 and 3
Abstract
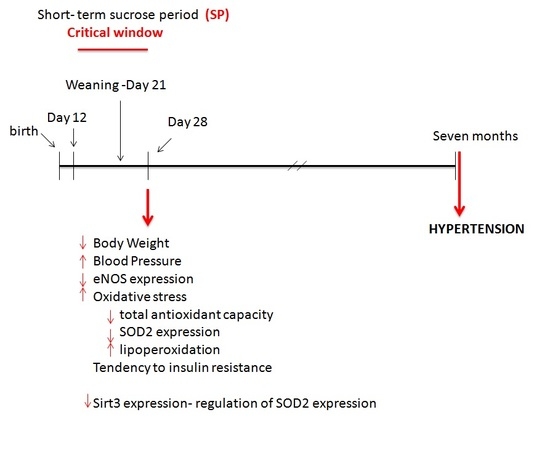
:1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Groups and General Characteristics of the Experimental Groups
2.2. Blood Pressure and Biochemical Determinations
2.3. Thoracic Aorta Homogenization.
2.4. Lipoperoxidation (LPO) and Total Antioxidant Capacity.
2.5. Endothelial Nitric Oxide Synthase, SOD1 and 2 and Sirt1 and 3 Immunoblotting
2.6. Statistical Analysis
3. Results
3.1. Body Variables, Food Intake, Blood Pressure and eNOS Expression
3.2. Changes in Insulin, Glucose and Insulin Resistance that Could Underlie Alterations in Blood Pressure
3.3. Changes in the Lipidic Profile that Could Underlie Changes in Vasoreactivity in Sucrose Period Rats
3.4. Oxidative Stress that Could Underlie Blood Pressure Alterations in the Sucrose Period Rats
3.5. Sirtuin 1 and Sirtuin 3 Expression
4. Discussion
4.1. Changes in Body Weight and Food Consumption
4.2. Mechanisms Underlying eNOS Expression
4.2.1. Possible Role of Insulin in Blood Pressure Regulation
4.2.2. Fatty Acids and the Expression of eNOS
4.2.3. Oxidative Stress and eNOS Expression
4.3. Substrate Availability and Epigenetic Regulated Expression of Hypertension Mediators
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| eNOS | endothelial nitric oxide synthase |
| FA | fatty acids |
| MS | metabolic syndrome |
| NO | nitric oxide |
| OA | oleic acid |
| OS | oxidative stress |
| RIA | radioimmunoassay |
| ROS | reactive oxygen species |
| SEM | standard errors of the mean |
| Sirt1-3 | sirtuins 1 and 3 |
| SOD | superoxide dismutase |
| TGs | triglycerides |
References
- Lawlor, D.A.; Smith, G.D. Early life determinants of adult blood pressure. Curr. Opin. Nephrol. Hypertens. 2005, 14, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.C.; Jackson, A.A. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low-protein diets. Clin. Sci. 1994, 86, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.L.; Weeks, D.L.; Rasch, R. Programming of adult blood pressure by maternal protein restriction: Role of nephrogenesis. Kidney Int. 2004, 65, 1339–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, B.T. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 2003, 41, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, M.F.; van Wijk, J.A.E.; Delemarre-van de Waal, H.A. Intrauterine growth restriction increases blood pressure and central pulse pressure measured with telemetry in aging rats. J. Hypertens. 2006, 24, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, A.M.; Matthews, P.A.; Jansen, E.; Taylor, P.D.; Poston, L. Sucrose feeding in mouse pregnancy leads to hypertension, and sex-linked obesity and insulin resistance in female offspring. Front. Physiol. 2013, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.J.; Wong, D.L.; Henderson, R.; Curtis, K.S.; Smith, J.C. High dietary NaCl early in development enhances mean arterial pressure of adult rats. Physiol. Behav. 2000, 71, 173–181. [Google Scholar] [CrossRef]
- Porter, J.P.; King, S.H.; Honeycut, A.D. Prenatal high-salt diet in the Sprague-Dawley rat programs blood pressure and heart rate hyperresponsiveness to stress in adult female offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R334–R342. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Romero, M.; Castrejón-Téllez, V.; Pérez-Torres, I.; Rubio-Ruiz, M.E.; Carreón-Torres, E.; Díaz-Díaz, E.; del Valle-Mondragón, L.; Guarner-Lans, V. Short-Term Exposure to High Sucrose Levels near Weaning Has a Similar Long-Lasting Effect on Hypertension as a Long-Term Exposure in Rats. Nutrients 2018, 10, 728. [Google Scholar] [CrossRef]
- Hernández-Díaz, A.; Arana-Martínez, J.C.; Carbó, R.; Espinosa-Cervantes, R.; Sánchez-Muñoz, F. Omentin: Role in insulin resistance, inflammation and cardiovascular protection. Arch. Cardiol. Mex. 2016, 86, 233–243. [Google Scholar] [CrossRef]
- Gremmels, H.; Bevers, L.M.; Fledderus, J.O.; Braam, B.; van Zonneveld, A.J.; Verhaar, M.C.; Joles, J.A. Oleic acid increases mitochondrial reactive oxygen species production and decreases endothelial nitric oxide synthase activity in cultured endotelial cells. Eur. J. Pharmacol. 2015, 751, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Zúñiga Muñoz, A.; Beltrán-Rodríguez, U.; Díaz-Díaz, E.; Martínez-Memije, R.; Guarner Lans, V. Modification of the liver fatty acids by Hibiscus sabdariffa Linnaeus (Malvaceae) infusion, its possible effect on vascular reactivity in a metabolic syndrome model. Clin. Exp. Hypertens. 2014, 36, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Martín del Campo, C.M.; Martínez-Rosas, M.; Guarner-Lans, V. Epigenetics of Subcellular Structure Functioning in the Origin of Risk or Resilience to Comorbidity of Neuropsychiatric and Cardiometabolic Disorders. Int. J. Mol. Sci. 2018, 19, 1456. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, P.J.; Sukumar, A.N.; Marsden, P.A. Epigenetics of Cardiovascular Disease—A New “Beat” in Coronary Artery Disease. Med. Epigenet. 2014, 2, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Sadoshima, J. The role of sirtuins in cardiac disease. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1375–H1389. [Google Scholar] [CrossRef] [PubMed]
- Farghali, H.; Kutinová Canoová, N.; Lekic, N. Resveratrol and Related Compounds as Antioxidants with an Allosteric Mechanism of Action in Epigenetic Drug Targets. Physiol. Res. 2013, 62, 1–13. [Google Scholar]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Ruíz, M.E.; Del Valle-Mondragón, L.; Castrejón-Tellez, V.; Carre’on-Torres, E.; Díaz-Díaz, E.; Guarner-Lans, V. Angiotensin II and 1–7 during aging in metabolic syndrome rats. Expression of AT1, AT2 and Mas receptors in abdominal white adipose tissue. Peptides 2014, 57, 101–108. [Google Scholar] [CrossRef]
- Pérez Méndez, O.; García Hernández, L. High-Density Lipoproteins (HDL) size and composition are modified in the rat by a diet supplemented with ‘Hass’ avocad o (Persea americana Miller). Arch. Cardiol. Mex. 2007, 77, 17–24. [Google Scholar]
- Akondi, R.N.; Trexler, R.V.; Pfiffner, S.M.; Mouser, P.J.; Sharma, S. Modified Lipid Extraction Methods for Deep Subsurface Shale. Front. Microbiol. 2017, 8, 1408. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Roque, P.; El Hafidi, M.; Diaz-Diaz, E.; Baños, G. Association of renal damage and oxidative stress in a rat model of metabolic syndrome. Influence of gender. Free Radic. Res. 2009, 43, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Harder, T.; Franke, K.; Kohlhoff, R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care 2002, 25, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sampson, D.A.; Hunsaker, H.A.; Jansen, G.R. Dietary protein quality, protein quantity and food intake: Effects on lactation and on protein synthesis and tissue composition in mammary tissue and liver in rats. J. Nutr. 1986, 116, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Lemosquet, S.; Delamaire, E.; Lapierre, H.; Blum, J.W.; Peyraud, J.L. Effects of glucose, propionic acid, and nonessential amino acids on glucose metabolism and milk yield in Holstein dairy cows. J. Dairy Sci. 2009, 92, 3244–3257. [Google Scholar] [CrossRef] [PubMed]
- Geursen, A.; Carne, A.; Grigor, M.R. Protein synthesis in mammary acini isolated from lactating rats: Effect of maternal diet. J. Nutr. 1987, 117, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Villegas, L.A.; Tovar, A.R.; López-Barradas, A.M.; Torres, N. The dietary protein/carbohydrate ratio differentially modifies lipogenesis and protein synthesis in the mammary gland, liver and adipose tissue during gestation and lactation. PLoS ONE 2013, 8, e69338. [Google Scholar] [CrossRef]
- Choi, C.S.; Kim, P.; Park, J.H.; Gonzales, E.L.; Kim, K.C.; Cho, K.S.; Ko, M.J.; Yang, S.M.; Seung, H.; Han, S.H.; et al. High sucrose consumption during pregnancy induced ADHD-like behavioral phenotypes in mice offspring. J. Nutr. Biochem. 2015, 26, 1520–1526. [Google Scholar] [CrossRef]
- Millichap, J.G.; Yee, M.M. The diet factor in attention-deficit/hyperactivity disorder. Pediatrics 2012, 129, 330–337. [Google Scholar] [CrossRef]
- Kanarek, R.B. Does sucrose or aspartame cause hyperactivity in children? Nutr. Rev. 1994, 52, 173–175. [Google Scholar] [CrossRef]
- Duclos, M.; Bouchet, M.; Vettier, A.; Richard, D. Genetic differences in hypothalamic-pituitary-adrenal axis activity and food restriction-induced hyperactivity in three inbred strains of rats. J. Neuroendocrinol. 2005, 17, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.; Gatti, C.; Bessière, B.; Mormède, P. Tonic and phasic effects of corticosterone on food restriction-induced hyperactivity in rats. Psychoneuroendocrinology 2009, 34, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.; Ouerdani, A.; Mormède, P.; Konsman, J.P. Food restriction-induced hyperactivity: Addiction or adaptation to famine? Psychoneuroendocrinology 2013, 38, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.C.; Herbst, K.; Sanabria, F. Characterizing operant hyperactivity in the Spontaneously Hypertensive Rat. Behav. Brain Funct. 2012, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Quon, M.J. Insulin action and insulin resistance in vascular endothelium. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 523–530. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, M.A.; Chávez-Salgado, J.; Peñeda-Flores, A.; Zapata, E.; Masso, F.; El-Hafidi, M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1198–E1207. [Google Scholar] [CrossRef]
- Drew, B.G.; Fidge, N.H.; Gallon-Beaumier, G.; Kemp, B.E.; Kingwell, B.A. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. PNAS 2004, 101, 6999–7004. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Hernandez-Morante, J.J.; Tébar, F.J.; Zamora, S. Relation between degree of obesity and site-specific adipose tissue fatty acid composition in a Mediterranean population. Nutrition 2011, 27, 170–176. [Google Scholar] [CrossRef]
- De Lima, T.M.; de Sa Lima, L.; Scavone, C.; Curi, R. Fatty acid control of nitric oxideproduction by macrophages. FEBS. Lett. 2006, 580, 3287–3295. [Google Scholar] [CrossRef]
- Vila, L. Cyclooxygenase and 5-lipoxygenase pathways in the vessel wall: Rolein atherosclerosis. Med. Res. Rev. 2004, 24, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Cury-Boaventura, M.F.; Gorjão, R.; De Lima, T.M.; Newsholme, P.; Curi, R. Comparative toxicity of oleic and linoleic acid on human lymphocytes. Life Sci. 2006, 78, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Kim, Y.M.; Song, H.S.; Park, K.Y.; Kim, Y.M.; Kim, M.S.; Pak, Y.K.; Lee, I.K.; Lee, J.D.; Park, S.-J.; et al. Oleic acid induces endothelin-1 expression through activation of protein kinase C and NF-kappa B. Biochem. Biophys. Res. Commun. 2003, 303, 891–995. [Google Scholar] [CrossRef]
- El Hafidi, M.; Valdez, R.; Baños, G. Possible relationship between altered fatty acid composition of serum, platelets, and aorta and hypertension induced by sugar feeding in rats. Clin. Exp. Hypertens. 2000, 22, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Arachidonic acid and eicosanoids as targets and effectors in second messenger interactions. Prostaglandins Leukot. Essent. Fatty Acids 1995, 53, 239–524. [Google Scholar] [CrossRef]
- Han, F.; Guo, Y.; Xu, L.; Hou, N.; Han, F.; Sun, X. Induction of haemeoxygenase-1 Directly Improves Endothelial Function in Isolated Aortas from Obese Rats through the Ampk-Pi3k/Akt-Enos Pathway. Cell Physiol. Biochem. 2015, 36, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Rupp, H.; Turcani, M.; Ohkubo, T.; Maisch, B.; Brilla, C. Dietary linolenic acid mediated increase in vascular prostacyclin formation. Mol. Cell. Biochem. 1996, 162, 59–64. [Google Scholar] [CrossRef]
- Das, U.N. A defect in D6 and D5 desaturases may be a factor in the initiationand progression of insulin resistance, the metabolic syndrome and ischemic heart disease in South Asians. Lipids Health Dis. 2010, 9, 130. [Google Scholar] [CrossRef]
- Verlengia, R.; Gorjão, R.; Kanunfre, C.C.; Bordin, S.; De Lima, T.M.; Newsholme, P.; Curi, R.; Lima, T.M. Genes regulated by arachidonic and oleic acids in Raji cells. Lipids 2003, 38, 1157–1165. [Google Scholar] [CrossRef]
- Priante, G.; Musacchio, E.; Pagnin, E.; Calò, L.A.; Baggio, B. Specific effect to arachidonic acid on inducible nitric oxide synthase mRNA expression in human osteoblastic cells. Clin. Sci. (Lond.) 2005, 109, 177–182. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.B.; Siwik, D.A.; Xiao, L.; Pimentel, D.R.; Singh, K.; Colucci, W.S. Role of oxidative stress in myocardial hypertrophy and failure. J. Mol. Cell. Cardiol. 2002, 34, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Jeroudi, M.O.; Patel, B.S.; Aruoma, O.I.; Halliwell, B.; Lai, E.K.; McCay, P.B. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion: Evidence that myocardial ‘stunning’is a manifestation of reperfusion injury. Circ. Res. 1989, 65, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Charniot, J.C.; Bonnefont-Rousselot, D.; Albertini, J.P.; Zerhouni, K.; Dever, S.; Richard, I.; Nataf, P.; Pavie, A.; Monsuez, Je.; Delattre, J.; et al. Oxidative stress implication in a new ex-vivo cardiac concordant xenotransplantatio model. Free Radic. Res. 2007, 41, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.O.; Etienne-Selloum, N.; Madeira, S.V.F.; Sarr, M.; Walter, A.; Dal-Ros, S.; Schott, C.; Chataigneau, T.; Schini-Kerth, V.B. Endothelium-derived contracting factors mediate the Ang II induced endothelial dysfunction in the rat aorta: Preventive effect of red wine polyphenols. Pflug. Arch. Eur. J. Physiol. 2010, 459, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Lasségue, B.; Ushio-Fukai, M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscl. Thromb. Vasc. Biol. 2000, 20, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Small, H.Y.; Migliarino, S.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Hypertension: Focus on autoimmunity and oxidative stress. Free Radic. Biol. Med. 2018, 125, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Serpillon, S.; Floyd, B.C.; Gupte, R.S.; George, S.; Kozicky, M.; Neito, V.; Recchia, F.; Stanley, W.; Wolin, M.S.; Gupte, S.A.; et al. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose- 6-phosphate dehydrogenase-derived NADPH. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H153–H162. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef]
- Murdoch, C.E.; Zhang, M.; Cave, A.C.; Shah, A.M. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc. Res. 2006, 71, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Minhas, K.M.; Saraiva, R.M.; Schuleri, K.H.; Lehrke, S.; Zheng, M.; Saliaris, A.P.; Berry, C.E.; Vandegaer, K.M.; Li, D.; Hare, J.M. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ. Res. 2006, 98, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Tsutsui, H.; Kinugawa, S.; Utsumi, H.; Kang, D.; Hattori, N.; Uchida, K.; Arimura, K.-I.; Egashira, K.; Takeshita, A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res. 1999, 85, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Van Der Laarse, A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol. Cell. Biochem. 2010, 333, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Champion, H.C.; Li, M.; Ren, S.; Rodriguez, E.R.; Tavazzi, B.; Lazzarino, G.; Paolocci, N.; Gabrielson, K.L.; Wang, Y.; et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Investig. 2005, 115, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, W.F.; Paolocci, N.; St. John, M.E.; Skaf, M.W.; Stewart, G.C.; Xie, J.S.; Harrison, R.W.; Zeichner, J.; Mudrick, D.; Marbán, E.; et al. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechano energetic uncoupling in the failing heart. Circ. Res. 2002, 90, 297–304. [Google Scholar] [CrossRef] [PubMed]
- White, C.N.; Liu, C.C.; Garcia, A.; Hamilton, E.J.; Chia, K.K.; Figtree, G.A.; Rasmussen, H.H. Activation of cAMP dependent signaling induces oxidative modification of the cardiac Na+-K+ pump and inhibits its activity. J. Biol. Chem. 2010, 285, 13712–13720. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Ann. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Wallace, D.C.; Fan, W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion 2010, 10, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Hitchler, M.J.; Domann, F.E. Metabolic defects provide a spark for the epigenetic switch in cancer. Free Radic. Biol. Med. 2009, 47, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Cyr, A.R.; Domann, F.E. The redox basis of epigenetic modifications: From mechanisms to functional consequences. Antioxid. Redox Signal. 2011, 15, 551–589. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Bultman, S.J. Metaboloepigenetics: Interrelationships between energy metabolism and epigenetic control of gene expression. J. Cell Physiol. 2012, 227, 3169–3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]







| Body Variables | Control | Sucrose Period |
|---|---|---|
| Body Weight (g) | 127.5 ± 1.9 | 107.6 ± 2.3 a |
| Visceral Adipose Tissue (mg) | 451.3 ± 0.05 | 461.3 ± 0.06 a |
| Water intake (mL/day) | 34.25 ± 0.98 | 20.75 ± 0.85 a |
| kcal equivalents | - | 24.9 ± 1.03 a |
| Food intake (g/day) | 15.17 ± 0.96 | 8.25 ± 0.58 a |
| kcal equivalents | 23.74 ± 1.5 | 12.91 ± 0.91 a |
| Total kcal | 23.74 ± 1.5 | 37.81 ± 1.5 a |
| Variables | Control | Sucrose Period |
|---|---|---|
| Glucose (mg/dL) | 62.41 ± 3.9 | 58.90 ± 3.3 |
| Insulin (µU/mL) | 0.927 ± 02 | 0.57 ± 01 |
| HOMA-IR | 0.185 ± 0.04 | 0.089 ± 0.02 |
| Lipidic Profile | Control | Sucrose Period |
|---|---|---|
| Triglycerides (mg/dL) | 75.01 ± 4.55 | 119.73 ± 11.33 a |
| Total cholesterol (mg/dL) | 84.38 ± 4.95 | 81.49 ± 3.51 |
| C-HDL (mg/dL) | 25.31 ± 1.13 | 18.80 ± 1.62 a |
| Non-C HDL (mg/dL) | 59.07 ± 4.28 | 62.69 ± 4.50 |
| Fatty Acid (%) | Control | Sucrose Period |
|---|---|---|
| Palmitic | 27.18 ± 1.42 | 29.77 ± 0.54 |
| Stearic | 13.25 ± 0.55 | 10.70 ± 0.54 a |
| Oleic | 17.88 ± 2.69 | 22.93 ± 0.77 a |
| Linoleic | 15.18 ± 0.69 | 12.19 ± 0.40 a |
| Arachidonic | 8.55 ± 0.70 | 7.40 ± 0.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrejón-Téllez, V.; Villegas-Romero, M.; Pérez-Torres, I.; Zarco, G.; Rubio-Ruiz, M.E.; Carreón-Torres, E.; Díaz-Díaz, E.; Grimaldo, O.E.; Guarner-Lans, V. Effect of Sucrose Ingestion at the End of a Critical Window that Increases Hypertension Susceptibility on Peripheral Mechanisms Regulating Blood Pressure in Rats. Role of Sirtuins 1 and 3. Nutrients 2019, 11, 309. https://doi.org/10.3390/nu11020309
Castrejón-Téllez V, Villegas-Romero M, Pérez-Torres I, Zarco G, Rubio-Ruiz ME, Carreón-Torres E, Díaz-Díaz E, Grimaldo OE, Guarner-Lans V. Effect of Sucrose Ingestion at the End of a Critical Window that Increases Hypertension Susceptibility on Peripheral Mechanisms Regulating Blood Pressure in Rats. Role of Sirtuins 1 and 3. Nutrients. 2019; 11(2):309. https://doi.org/10.3390/nu11020309
Chicago/Turabian StyleCastrejón-Téllez, Vicente, Mariana Villegas-Romero, Israel Pérez-Torres, Gabriela Zarco, María Esther Rubio-Ruiz, Elizabeth Carreón-Torres, Eulises Díaz-Díaz, Oscar Emanuel Grimaldo, and Verónica Guarner-Lans. 2019. "Effect of Sucrose Ingestion at the End of a Critical Window that Increases Hypertension Susceptibility on Peripheral Mechanisms Regulating Blood Pressure in Rats. Role of Sirtuins 1 and 3" Nutrients 11, no. 2: 309. https://doi.org/10.3390/nu11020309
APA StyleCastrejón-Téllez, V., Villegas-Romero, M., Pérez-Torres, I., Zarco, G., Rubio-Ruiz, M. E., Carreón-Torres, E., Díaz-Díaz, E., Grimaldo, O. E., & Guarner-Lans, V. (2019). Effect of Sucrose Ingestion at the End of a Critical Window that Increases Hypertension Susceptibility on Peripheral Mechanisms Regulating Blood Pressure in Rats. Role of Sirtuins 1 and 3. Nutrients, 11(2), 309. https://doi.org/10.3390/nu11020309