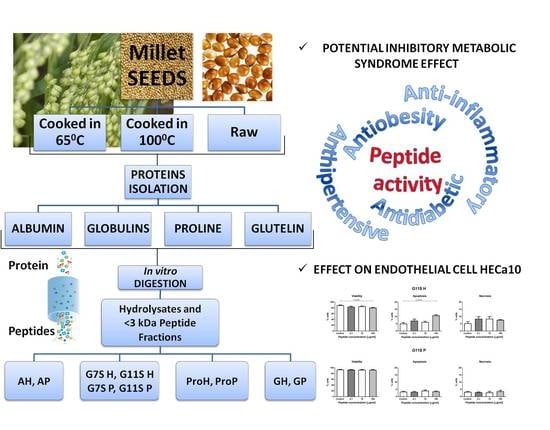
Different Temperature Treatments of Millet Grains Affect the Biological Activity of Protein Hydrolyzates and Peptide Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Millet Grain Heating
2.3. Protein Fractionation
2.4. In Vitro Hydrolysis of Proteins and Preparation of the Peptide Fraction
2.5. Degree of Hydrolysis (DH)
2.6. Potential Bioaccessibility and Bioavailability of Peptides Obtained from Millet Proteins
- PAC = Cph/Cpb
- Cph–peptide content in the hydrolyzate
- Cpb–peptide content in the sample before hydrolysis
- PAV = Cpa/Cph
- Cpa–peptide content after the absorption process
- Cph–peptide content in the hydrolyzate
2.7. Enzyme Inhibitory Activity Assay
2.7.1. Angiotensin-Converting Enzyme (ACE) Inhibitory Assay
- ACE inhibition (%) = [1 − ((A1 − A2)/A3)] × 100, where:
- A1 is the absorbance of the sample with ACE and the inhibitor,
- A2 is the absorbance of the sample with inhibitor without ACE,
- A3 is the absorbance of the sample with ACE and without the inhibitor.
2.7.2. α-Amylase Inhibitory Assay
2.7.3. α-Glucosidase Inhibitory Assay (αGIA)
2.8. Effect of Protein Hydrolyzates and Peptide Fractions on the Metabolism of Endothelial Cells (HECa10 Line)
2.8.1. MTT Test
2.8.2. NR Test
2.8.3. Cell Viability and Type of Cell Death
2.8.4. Cell Cycle
2.9. Peptide Separation by Gel Filtration Chromatography
2.10. Identification of Peptides
2.11. Statistical Analysis
3. Results
3.1. Degree of Hydrolysis and Potential Bioaccessibility (PAC) and Bioavailability (PAV) of Peptides
3.2. Inhibition of Metabolic Syndrome Enzymes
3.3. Effect of Protein Hydrolyzates and Peptide Fractions on HECa 10 Cells
3.3.1. Effect of the Prolamin Hydrolyzate (PRO H) and Peptide Fraction (PRO P) from Control Prolamin–MTT and NR Tests
3.3.2. Effect of the Hydrolyzate (G11S H) and Peptide Fraction (G11S P) from Globulin Obtained from Millet in the 65 °C Treatment
3.3.3. Effect of the Hydrolyzate (PH) and Peptide Fraction (PP) from Prolamin after the 100 °C Treatment
3.4. Characteristics and Identification of Peptide Fractions with Molecular Mass Under 3.0 kDa with the Highest Potential Inhibitory Activity Towards Enzymes Involved in Metabolic Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, A. Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Technol. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Capriles, V.D.; Arêas, J.A.G. Novel approaches in gluten-free breadmaking: Interface between food science, nutrition, and health. Compr. Rev. Food Sci. Food Saf. 2014, 13, 871–890. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Singh, E. Potential of Millets: Nutrients Composition and Health Benefits. J. Sci. Innov. Res. JSIR 2016, 5, 46–50. [Google Scholar]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Duan, W.; Ren, X.; Wang, C.; Pan, Z. Effect of foxtail millet protein hydrolysates on lowering blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2016, 56, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 17, 3143–3421.
- Kunyanga, C.N.; Imungi, J.K.; Okoth, M.W.; Biesalski, H.K.; Vadivel, V. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT—Food Sci. Technol. 2012, 45, 269–276. [Google Scholar] [CrossRef]
- Garcia, V.; Joseph, G.; Shkolnik, B.; Ding, Y.; Zhang, F.F.; Gotlinger, K.; Falck, J.R.; Dakarapu, R.; Capdevila, J.H.; Bernstein, K.E.; et al. Angiotensin II receptor blockade or deletion of vascular endothelial ACE does not prevent vascular dysfunction and remodeling in 20-HETE-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R71–R78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas-Peralta, M.; Jiménez-Genchi, G.M. New Challenges for Treatment in Hypertension. Arch. Med. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Marcone, S.; Haughton, K.; Simpson, P.J.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides inhibit human endothelial-monocyte interactions via PPAR-γ dependent regulation of NF-κB. J. Inflamm. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Malinda, K.M.; Sidhu, G.S.; Banaudha, K.K.; Gaddipati, J.P.; Maheshwari, R.K.; Goldstein, A.L.; Kleinman, H.K.; Malinda, K.M.; Sidhu, G.S.; Banaudha, K.K.; et al. Thymosin α 1 Stimulates Endothelial Cell Migration, Angiogenesis, and Wound Healing. J. Immunol. 2018, 160, 1001–1006. [Google Scholar]
- Jakala, P.; Pere, E.; Lehtinen, R.; Turpeinen, A.; Korpela, R.; Vapaatalo, H. Cardiovascular activity of milk casein-derived tripeptides and plant sterols in spontaneously hypertensive rats. J. Physiol. Pharmacol. 2009, 60, 11–20. [Google Scholar] [PubMed]
- Silva-Sánchez, C.; de la Rosa, A.P.B.; León-Galván, M.F.; de Lumen, B.O.; de León-Rodríguez, A.; de Mejía, E.G. Bioactive peptides in amaranth seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U. Identification of potential inhibitory peptides of enzymes involved in the metabolic syndrome obtained by simulated gastrointestinal digestion of fermented bean (Phaseolus vulgaris L.) seeds. Food Res. Int. 2017, 100, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 2002, 27, 1256–1262. [Google Scholar] [CrossRef]
- Gawlik-dziki, U.; Dziki, D.; Swieca, M.; Se, Ł. Bread enriched with Chenopodium quinoa leaves powder e The procedures for assessing the fortification efficiency. LWT—Food Sci. Technol. 2015, 62, 1226–1234. [Google Scholar] [CrossRef]
- Świeca, M.; Baraniak, B.; Gawlik-Dziki, U. In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013, 138, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Świeca, M.; Gawlik-Dziki, U.; Dziki, D. Nutritional potential and inhibitory activity of bread fortified with green coffee beans against enzymes involved in metabolic syndrome pathogenesis. LWT—Food Sci. Technol. 2018, 95, 78–89. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004–2013. [Google Scholar]
- Rokicki, D.; Zdanowski, R.; Lewicki, S.; Leśniak, M.; Suska, M.; Wojdat, E.; Skopińska-Rózewska, E.; Skopiński, P. Inhibition of proliferation, migration and invasiveness of endothelial murine cells culture induced by resveratrol. Cent. Eur. J. Immunol. 2014, 39, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Leśniak, M.; Zdanowski, R.; Suska, M.; Brewczyńska, A.; Stankiewicz, W.; Kloc, M.; Kubiak, J.Z.; Lewicki, S. Effects of Hexachlorophene, a Chemical Accumulating in Adipose Tissue, on Mouse and Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2018, 15, 211–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubczyk, A.; Karaś, M.; Baraniak, B.; Pietrzak, M. The impact of fermentation and in vitro digestion on formation angiotensin converting enzyme (ACE) inhibitory peptides from pea proteins. Food Chem. 2013, 141, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.J.; Goodwin, C.J.; Holt, S.J. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul. 1995, 5, 69–84. [Google Scholar] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyż, J. The influence of protein-flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Cheng, L.; Li, X.; Zhang, D.; Wu, G.; Zhang, H.; Wang, L.; Qian, H.; Wang, Y. Effect of cooking methods on solubility and nutrition quality of brown rice powder. Food Chem. 2019, 274, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of intestinal epithelial cells properties and functions by amino acids. Biomed. Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef] [PubMed]
- Defagó, M.D.; Elorriaga, N.; Irazola, V.E.; Rubinstein, A.L. Influence of Food Patterns on Endothelial Biomarkers: A Systematic Review. J. Clin. Hypertens. 2014, 16, 907–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Der Plancken, I.V.; Remoortere, M.V.; Loey, A.V.; Hendrick, M.E. Heat-Induced Changes in the Susceptibility of Egg White Proteins to Enzymatic Hydrolysis: A Kinetic Study. J. Agric. Food Chem. 2003, 51, 3819–3823. [Google Scholar] [CrossRef] [PubMed]
- Uraipong, C.; Zhao, J. in vitro digestion of rice bran proteins produces peptides with potent inhibitory effects on α-glucosidase and angiotensin I converting enzyme. J. Sci. Food Agric. 2017, 98, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Zeng, H.; Bi, G.; Claude, F.; Feng, B. Heat-pretreatment and enzymolysis behavior of the lotus seed protein. Food Chem. 2016, 201, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Auwal, M.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R.M. Biomedicine & Pharmacotherapy Rational in silico design of novel α -glucosidase inhibitory peptides and in vitro evaluation of promising candidates. Biomed. Pharmacother. 2018, 107, 234–242. [Google Scholar]
- Maestri, E.; Pavlicevic, M.; Montorsi, M.; Marmiroli, N. Meta-Analysis for Correlating Structure of Bioactive Peptides in Foods of Animal Origin with Regard to Effect and Stability. Compr. Rev. Food Sci. Food Saf. 2018, 18, 3–30. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Liu, C.; Zheng, X.; Liu, B. Enhancing antioxidant activity and antiproliferation of wheat bran through steam flash explosion. J. Food Sci. Technol. 2016, 53, 3028–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.Y.; Osada, K.; Ito, Y.; Nagasawa, T.; Choi, M.R.; Nishizawa, N. Effects of dietary protein of Korean foxtail millet on plasma adiponectin, HDL-cholesterol, and insulin levels in genetically type 2 diabetic mice. Biosci. Biotechnol. Biochem. 2005, 69, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, G.; Yang, J.; Zheng, R.; Jiang, L.; Bao, P. The association between metabolic syndrome and vascular endothelial dysfunction in adolescents. Exp. Ther. Med. 2013, 5, 1663–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janus, A.; Szahidewicz-Krupska, E.; Mazur, G.; Doroszko, A. Insulin Resistance and Endothelial Dysfunction Constitute a Common Therapeutic Target in Cardiometabolic Disorders. Mediat. Inflamm. 2016, 2016, 3634948. [Google Scholar] [CrossRef] [PubMed]
- Carcamo-Orive, I.; Huang, N.F.; Quertermous, T.; Knowles, J.W. Induced Pluripotent Stem Cell-Derived Endothelial Cells in Insulin Resistance and Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Baraniak, B. Angiotensin I Converting Enzyme Inhibitory Peptides Obtained after in vitro Hydrolysis of Pea (Pisum sativum var. Bajka) Globulins. Biomed. Res. Int. 2014, 2014, 438459. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, A.; Matheus, D.M.; Righeti, L.; Tannus, M.; Cobas, R.A.; Palma, C.C.S.; Negrato, C.A.; Gomes, M.D.B. Impact of Diabetes on Cardiovascular Disease: An Update. Int. J. Hypertens. 2013, 2013, 653789. [Google Scholar]
- Yamagishi, S. Cardiovascular disease in recent onset diabetes mellitus diabetes. J. Cardiol. 2011, 57, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Siow, H.L.; Choi, S.B.; Gan, C.Y. Structure–activity studies of protease activating, lipase inhibiting, bile acid binding and cholesterol-lowering effects of pre-screened cumin seed bioactive peptides. J. Funct. Foods 2016, 27, 600–611. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Reza, M. ScienceDirect Production of antioxidant and ACE-inhibitory peptides from Kluyveromyces marxianus protein hydrolysates: Purification and molecular docking. J. Food Drug Anal. 2017, 26, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, A.K.; Shastri, V.; Madhu, M.K.; Sharma, V.K. Prediction of anti-inflammatory proteins/peptides: An insilico approach. J. Transl. Med. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fang, L.; Min, W.; Liu, J.; Li, H. Exploration of the molecular interactions between angiotensin-I-converting enzyme (ACE) and the inhibitory peptides derived from hazelnut (Corylus heterophylla Fisch). Food Chem. 2018, 245, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, Y.; Soon, T.; Gan, C. Enzyme and Microbial Technology Screening and identification of five peptides from pinto bean with inhibitory activities against α-amylase using phage display technique. Enzym. Microb. Technol. 2016, 89, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, N.; Wang, W.; Wang, J.; Zhu, Z.; Li, X. Peptides Molecular mechanisms of novel peptides from silkworm pupae that inhibit α-glucosidase. Peptides 2016, 76, 45–50. [Google Scholar] [CrossRef] [PubMed]










| Enzyme | α-Amylase | Pepsin | Pancreatin | PAC | PAV | |
|---|---|---|---|---|---|---|
| Protein | ||||||
| 65 °C | ||||||
| Albumin | 69.24 ± 1.32 Aa | 85.36 ± 1.74 Ba | 91.52 ± 1.55 CAb | 1.06 | 0.12 | |
| Globulin 7S | 59.99 ± 1.22 Aa | 63.03 ± 1.61 Aa | 86.69 ± 1.47 Ba | 1.96 | 0.40 | |
| Globulin 11S | 26.46 ± 0.89 Aa | 30.81 ± 1.11 Ba | 34.47 ± 1.01 Ca | 6.47 | 2.12 | |
| Prolamin | 48.67 ± 2.01 Aa | 53.77 ± 1.37 Ba | 62.23 ± 1.87 Ca | 2.74 | 1.24 | |
| Glutelin | 41.46 ± 1.44 Aa | 43.21 ± 1.78 Aa | 57.09 ± 1.31 Ba | 1.94 | 0.27 | |
| 100 °C | ||||||
| Albumin | 56.31 ± 2.17 Ab | 76.16 ± 1.57 Bb | 89.20 ± 2.07 Ca | 1.14 | 0.14 | |
| Globulin 7S | 29.26 ± 0.69 Ab | 30.29 ± 0.77 Ab | 46.26 ± 1.69 Bb | 2.92 | 0.36 | |
| Globulin 11S | 13.78 ± 0.98 Ab | 20.47 ± 0.66 Bb | 47.68 ± 1.78 Cb | 23.89 | 0.57 | |
| Prolamin | 13.19 ± 0.36 Ab | 20.51 ± 0.91 Bb | 47.52 ± 1.21 Cb | 9.56 | 0.56 | |
| Glutelin | 62.34 ± 1.25 Ab | 64.59 ± 1.24 Ab | 88.47 ± 1.74 Bb | 3.73 | 0.24 | |
| C | ||||||
| Albumin | 54.00 ± 1.88 Ab | 62.04 ± 1.70 Bc | 94.45 ± 2.07 Cb | 1.92 | 0.11 | |
| Globulin 7S | 66.89 ± 1.98 Ac | 78.47 ± 2.00 Ac | 98.33 ± 1.21 Ac | 1.18 | 0.15 | |
| Globulin 11S | 34.51 ± 1.14 Ac | 38.07 ± 0.77 Bc | 38.56 ± 1.87 Bc | 1.18 | 0.21 | |
| Prolamin | 46.56 ± 1.22 Aa | 48.69 ± 1.01 Ac | 57.82 ± 1.29 Bc | 1.34 | 0.15 | |
| Glutelin | 58.08 ± 1.63 Ac | 60.12 ± 1.44 Ac | 81.26 ± 1.89 Bc | 1.79 | 0.14 | |
| Temperature | 65 °C | 100 °C | C | |
|---|---|---|---|---|
| Protein | ||||
| ACE | ||||
| Albumin | 3.25 ± 0.87 ABa | 2.40 ± 0.74 Aab | 4.73 ± 0.54 Ba | |
| Globulin 7S | 2.00 ± 0.01 Ab | 2.63 ± 0.36 Aa | 4.85 ± 0.44 Ba | |
| Globulin 11S | 0.44 ± 0.01 Ac | 1.50 ± 0.02 Bbc | 4.89 ± 0.21 Ca | |
| Prolamin | 1.24 ± 0.03 Ab | 1.38 ± 0.01 Ac | 3.52 ± 0.31 Bb | |
| Glutelin | 1.73 ± 0.12 Ab | 2.12 ± 0.17 Aabc | 6.39 ± 0.28 Bc | |
| α-amylase | ||||
| Albumin | 1.37 ± 0.02 A | 3.84 ± 0.34 Ba | 1.92 ± 0.01 Ca | |
| Globulin 7S | nd | 5.47 ± 0.11 Ab | 3.27 ± 0.51 Bb | |
| Globulin 11S | nd | 2.37 ± 0.08 Aa | 6.32 ± 0.44 Bc | |
| Prolamin | nd | 8.21 ± 1.17 Ac | 0.77 ± 0.01 Bd | |
| Glutelin | nd | 0.12 ± 0.01 Ad | 1.38 ±0.04 Bad | |
| α-glucosidase | ||||
| Albumin | 0.08 ± 0.001 Aa | 0.60 ± 0.013 Ba | 0.49 ± 0.011 Ca | |
| Globulin 7S | 0.58 ± 0.002 Ab | 0.89 ± 0.017 Bb | 1.46 ± 0.021 Cb | |
| Globulin 11S | 0.24 ± 0.003 Ac | 0.35 ± 0.018 Bc | 0.12 ± 0.001 Cc | |
| Prolamin | 0.06 ± 0.003 Ad | 0.51 ± 0.013 Bd | 1.13 ± 0.001 Cd | |
| Glutelin | 0.57 ± 0.012 Ab | 0.60 ± 0.015 Aa | nd | |
| Temperature | 65 °C | 100 °C | C | |
|---|---|---|---|---|
| Protein | ||||
| ACE | ||||
| Albumin | 0.41 ± 0.002 Aa | 0.45 ± 0.012 Aa | 0.83 ± 0.011 Ba | |
| Globulin 7S | 0.54 ± 0.013 Ab | 0.37 ± 0.011 Bb | 0.60 ± 0.022 Cb | |
| Globulin 11S | 0.38 ± 0.015 Aa | 0.65 ± 0.021 Bc | 0.79 ± 0.025 Ca | |
| Prolamin | 0.54 ± 0.016 Ab | 0.33 ± 0.001 Bd | 0.42 ± 0.010 ABc | |
| Glutelin | 0.66 ± 0.018 Ac | 0.63 ± 0.014 ABc | 0.61 ± 0.012 Bb | |
| α-amylase | ||||
| Albumin | nd | 0.24 ± 0.014 A | 0.39 ± 0.017 Ba | |
| Globulin 7S | nd | nd | 0.30 ± 0.001 b | |
| Globulin 11S | nd | nd | 0.44 ± 0.011 c | |
| Prolamin | nd | nd | 0.11 ± 0.002 d | |
| Glutelin | nd | nd | 0.67 ± 0.012 e | |
| α-glucosidase | ||||
| Albumin | 0.05 ± 0.004 Aa | 0.26 ± 0.022 Ba | 0.10 ± 0.001 Ca | |
| Globulin 7S | 0.22 ± 0.001 Ab | 0.29 ± 0.002 Bb | 0.14 ± 0.001 Cb | |
| Globulin 11S | 0.05 ± 0.001 Aa | nd | 0.09 ± 0.013 Ba | |
| Prolamin | 0.18 ± 0.003 Ac | 0.12 ± 0.001 Ba | nd | |
| Glutelin | 0.06 ± 0.003 Ad | 0.31 ± 0.002 Bd | nd | |
| Sample | ACE | α-Amylase | α-Glucosidase |
|---|---|---|---|
| Fraction from PRO P: | |||
| 1 | 4.82 ± 0.13 Aa | 43.56 ± 4.75 Ba | 91.38 ± 5.37 Ca |
| 2 | 23.61 ± 1.18 Ab | 49.73 ± 2.59 Bb | 87.69 ± 1.83 Ca |
| Fraction from G11S P: | |||
| I | 10.01 ± 0.51 Ac | 60.71 ± 5.05 Bc | 35.06 ± 6.03 Cb |
| II | 13.28 ± 0.16 Ad | 68.74 ± 2.09 Bb | 36.20 ± 3.11 Cb |
| Fraction from PP: | |||
| A | 82.02 ± 2.01 Ae | 127.96 ± 9.82 Bd | 128.38 ± 2.51 Bc |
| B | 31.27 ± 1.17 Af | 118.12 ±1.65 Be | 107.01 ± 2.22 Bd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Jęderka, K.; Lewicki, S.; Złotek, U. Different Temperature Treatments of Millet Grains Affect the Biological Activity of Protein Hydrolyzates and Peptide Fractions. Nutrients 2019, 11, 550. https://doi.org/10.3390/nu11030550
Karaś M, Jakubczyk A, Szymanowska U, Jęderka K, Lewicki S, Złotek U. Different Temperature Treatments of Millet Grains Affect the Biological Activity of Protein Hydrolyzates and Peptide Fractions. Nutrients. 2019; 11(3):550. https://doi.org/10.3390/nu11030550
Chicago/Turabian StyleKaraś, Monika, Anna Jakubczyk, Urszula Szymanowska, Krystyna Jęderka, Sławomir Lewicki, and Urszula Złotek. 2019. "Different Temperature Treatments of Millet Grains Affect the Biological Activity of Protein Hydrolyzates and Peptide Fractions" Nutrients 11, no. 3: 550. https://doi.org/10.3390/nu11030550
APA StyleKaraś, M., Jakubczyk, A., Szymanowska, U., Jęderka, K., Lewicki, S., & Złotek, U. (2019). Different Temperature Treatments of Millet Grains Affect the Biological Activity of Protein Hydrolyzates and Peptide Fractions. Nutrients, 11(3), 550. https://doi.org/10.3390/nu11030550