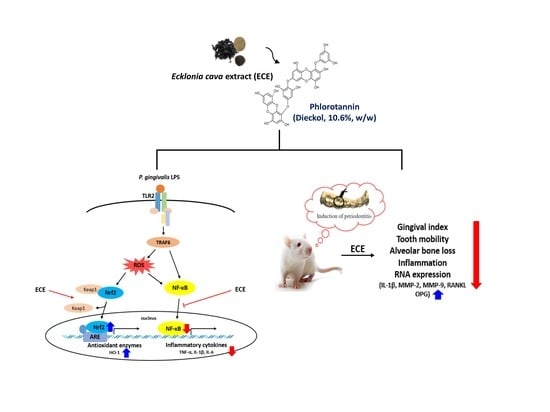
Anti-Inflammatory Effect of Ecklonia cava Extract on Porphyromonas gingivalis Lipopolysaccharide-Stimulated Macrophages and a Periodontitis Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Nitric Oxide, Prostaglandin E2, and Cyclooxygenase-2 Activity Measurement
2.4. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
2.5. Western Blotting Analyses
2.6. Animals and Induction of Experimental Periodontitis
2.7. Experimental Groups
2.8. Gingival Index and Tooth Mobility
2.9. Micro-Computerized Tomography
2.10. Histopathological Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of ECE on NO and PGE2 Production, and COX-2 Activity in P. gingivalis LPS-Stimulated RAW 264.7 Cells
3.2. Effect of ECE on Pro-Inflammatory Enzyme and Cytokine Gene Expressions in P. gingivalis LPS-Stimulated RAW 264.7 Cells
3.3. Effect of ECE on Induction HO-1 and Nuclear NF-κB Translocation in P. gingivalis LPS-Stimulated RAW 264.7 Cells
3.4. Effects of ECE on Gingival Index, Tooth Mobility, and Alveolar Bone Loss in Ligatured-Induced Periodontitis in Rats
3.5. Effects of ECE on Inflammation and Osteoclastogenesis Related Gene Expressions in Gingival Tissues
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beck, J.D.; Offenbacher, S. Systemic effects of periodontitis: Epidemiology of periodontal disease and cardiovascular disease. J. Periodontol. 2005, 76, 2089–2100. [Google Scholar] [CrossRef]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodonto-pathic pathogen overview. J. Immunol. Res. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol. 2000 2013, 62, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, D.J.; Kim, Y.H.; Kim, Y.H.; Kim, S.G.; Shon, K.J.; Choi, Y.W.; Lee, S.J. Upregulation of heme oxygenase-1 via PI3K/Akt and Nrf-2 signaling pathways mediates the anti-inflammatory activity of schisandrin in Porphyromonas gingivalis LPS-stimulated macrophages. Immunol. Lett. 2011, 139, 93–101. [Google Scholar] [CrossRef]
- Araujo, A.A.; Souza, T.O.; Moura, L.M.; Brito, G.A.C.; Aragao, K.S.; Araujo, L.S.; Medeiros, C.A.X.; Alves, M.S.C.F.; Araujo, R.F., Jr. Effect of telmisartan on levels of IL-1, TNF-α, down-regulated COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis model. J. Clin. Periodontol. 2013, 40, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ko, J.Y.; Oh, J.Y.; Kim, C.Y.; Lee, H.J.; Kim, J.; Jeon, Y.J. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014, 158, 433–437. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Jung, W.K.; Ahn, Y.W.; Lee, S.H.; Choi, Y.H.; Kim, S.K.; Yea, S.S.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; et al. Ecklonia cava ethanolic extracts inhibit lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in BV2 microglia via the MAP kinase and NF-κB pathways. Food Chem. Toxicol. 2009, 47, 410–417. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.S.; Choi, S.I.; Kim, G.H.; Imm, J.Y. Ecklonia cava extract containing dieckol suppresses RANKL-induced osteoclastogenesis via inhibition of MAP kinase/NF-κB pathway and induction of heme oxygenase-1. J. Microbiol. Biotechnol. 2019, 29, 11–20. [Google Scholar] [CrossRef]
- Lee, D.; Imm, J.Y. AMP kinase activation and inhibition of nuclear factor-kappa B (NF-κB) translocation contribute to the anti-inflammatory effect of tricin. J. Food Biochem. 2017, 41, e12293. [Google Scholar] [CrossRef]
- Kim, J.G.; Lim, D.W.; Cho, S.; Han, D.; Kim, Y.T. The Edible brown seaweed Ecklonia cava reduces hypersensitivity in postoperative and neuropathic pain models in rats. Molecules 2014, 19, 7669–7678. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, W. A comparative study of systemic subantimicrobial and topical treatment of minocycline in experimental periodontitis of rats. Arch. Oral Biol. 2006, 51, 794–803. [Google Scholar] [CrossRef]
- Cai, X.; Li, C.; Du, G.; Cao, Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J. Periodontal Res. 2008, 43, 14–21. [Google Scholar] [CrossRef]
- Aguirre, J.I.; Akhter, M.P.; Neuville, K.G.; Trcalek, C.R.; Leeper, A.M.; Williams, A.A.; Rivera, M.; Kesavalu, L.; Ke, H.Z.; Liu, M.; et al. Age-related periodontitis and alveolar bone loss in rice rats. Arch. Oral Biol. 2017, 73, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Ahn, G.; Lee, W.W.; Kang, M.C.; Kim, E.A.; Jeon, Y.J. Anti-inflammatory activity of phlorotannin-rich fermented Ecklonia cava processing by-product extract in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Appl. Phycol. 2013, 25, 1207–1213. [Google Scholar] [CrossRef]
- Martin, M.; Schifferle, R.E.; Cuesta, N.; Vogel, S.N.; Katz, J.; Michalek, S.M. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J. Immunol. 2003, 171, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Toshchakov, V.; Jones, B.W.; Lentschat, A.; Silva, A.; Perera, P.Y.; Thomas, K.; Cody, M.J.; Zhang, S.; Williams, B.R.G.; Major, J.; et al. TLR2 and TLR4 agonists stimulate unique repertoires of host resistance genes in murine macrophages: Interferon-β-dependent signaling in TLR4-mediated responses. J. Endotoxin Res. 2003, 9, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Tsatsanis, C.; Androulidaki, A.; Venihaki, M.; Margioris, A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006, 38, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Mestre, J.R.; Mackrell, P.J.; Rivadeneira, D.E.; Stapleton, P.P.; Tanabe, T.; Daly, J.M. Redundancy in the signaling pathways and promoter elements regulating cyclooxygenase-2 gene expression in endotoxin-treated macrophage/monocytic cells. J. Biol. Chem. 2001, 276, 3977–3982. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Rodiles, M.; Tiraloche, G.; Chadee, K. Lipopolysaccharide modulates cyclooxygenase-2 transcriptionally and posttranscriptionally in human macrophages independently from endogenous IL-1 beta and TNF-alpha. J. Immunol. 1999, 163, 963–969. [Google Scholar] [PubMed]
- Schrader, L.I.; Kinzenbaw, D.A.; Johnson, A.W.; Faraci, F.M.; Didion, S.P. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscl. Throm. Vas. 2007, 27, 2576–2581. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; Calabrese, L. The pivotal role of interleukin-1 in the clinical manifestations of rheumatoid arthritis. Rheumatology 2004, 43, iii2–iii9. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Hsu, W.L.; Chen, T.H.; Chou, T.C. Activation of Nrf2/HO-1 signaling pathway involves the anti-inflammatory activity of magnolol in Porphyromonas gingivalis lipopolysaccharide-stimulated mouse RAW 264.7 macrophages. Int. Immunopharmacol. 2015, 29, 770–778. [Google Scholar] [CrossRef]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Kwon, M.S.; Choi, J.W.; Shin, T.; No, H.K.; Choi, J.S.; Byun, D.S.; Kim, J.I.; Kim, H.R. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J. Agric. Food Chem. 2012, 60, 9120–9129. [Google Scholar] [CrossRef]
- Yayeh, T.; Im, E.J.; Kwon, T.H.; Roh, S.S.; Kim, S.; Kim, J.H.; Hong, S.B.; Cho, J.Y.; Park, N.H.; Rhee, M.H. Hemeoxygenase 1 partly mediates the anti-inflammatory effect of dieckol in lipopolysaccharide stimulated murine macrophages. Int. Immunopharmacol. 2014, 22, 51–58. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Hefti, A.F.; Jepsen, S.; Etienne, D.; Walker, C.; Bradshaw, M.H. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis: A review. J. Clin. Periodontol. 2004, 31, 697–707. [Google Scholar] [CrossRef]
- Goetze, S.; Hiernickel, C.; Elsner, P. Phototoxicity of doxycycline: A systematic review on clinical manifestations, frequency, cofactors, and prevention. Skin Pharmacol. Phys. 2017, 30, 76–80. [Google Scholar] [CrossRef]
- Smith, K.; Leyden, J.J. Safety of doxycycline and minocycline: A systematic review. Clin. Ther. 2005, 27, 1329–1342. [Google Scholar] [CrossRef]
- Chakravarti, A.; Raquil, M.A.; Tessier, P.; Poubelle, P.E. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood 2009, 114, 1633–1644. [Google Scholar] [CrossRef]
- Shin, H.C.; Hwang, H.J.; Kang, K.J.; Lee, B.H. An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch. Pharm. Res. 2006, 29, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Moon, I.S.; Choi, B.K.; Paik, J.W.; Kim, Y.S.; Choi, S.H.; Kim, C.K. Effects of sub-antimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitis. J. Periodontal Res. 2004, 39, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V. Host-response therapeutics for periodontal diseases. J. Periodontol. 2008, 79, 1592–1600. [Google Scholar] [CrossRef]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Crotti, T.; Smith, M.D.; Hirsch, R.; Soukoulis, S.; Weedon, H.; Capone, M.; Ahern, M.J.; Haynes, D. Receptor activator NF-κB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J. Periodontal Res. 2003, 38, 380–387. [Google Scholar] [CrossRef]






| Group | Gingival Index | Tooth Mobility |
|---|---|---|
| Normal control | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| Ligation control | 1.50 ± 0.55 a | 1.40 ± 0.55 a |
| Doxycycline 20 mg/kg | 0.50 ± 0.55 c | 0.40 ± 0.55 cd |
| ECE 100 mg/kg | 1.00 ± 0.00 b | 1.20 ± 0.45 ab |
| ECE 200 mg/kg | 1.00 ± 0.00 b | 1.00 ± 0.00 abc |
| ECE 400 mg/kg | 0.83 ± 0.41 bc | 0.60 ± 0.55 bcd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Choi, S.-I.; Kim, G.-H.; Imm, J.-Y. Anti-Inflammatory Effect of Ecklonia cava Extract on Porphyromonas gingivalis Lipopolysaccharide-Stimulated Macrophages and a Periodontitis Rat Model. Nutrients 2019, 11, 1143. https://doi.org/10.3390/nu11051143
Kim S, Choi S-I, Kim G-H, Imm J-Y. Anti-Inflammatory Effect of Ecklonia cava Extract on Porphyromonas gingivalis Lipopolysaccharide-Stimulated Macrophages and a Periodontitis Rat Model. Nutrients. 2019; 11(5):1143. https://doi.org/10.3390/nu11051143
Chicago/Turabian StyleKim, Seonyoung, Soo-Im Choi, Gun-Hee Kim, and Jee-Young Imm. 2019. "Anti-Inflammatory Effect of Ecklonia cava Extract on Porphyromonas gingivalis Lipopolysaccharide-Stimulated Macrophages and a Periodontitis Rat Model" Nutrients 11, no. 5: 1143. https://doi.org/10.3390/nu11051143
APA StyleKim, S., Choi, S.-I., Kim, G.-H., & Imm, J.-Y. (2019). Anti-Inflammatory Effect of Ecklonia cava Extract on Porphyromonas gingivalis Lipopolysaccharide-Stimulated Macrophages and a Periodontitis Rat Model. Nutrients, 11(5), 1143. https://doi.org/10.3390/nu11051143