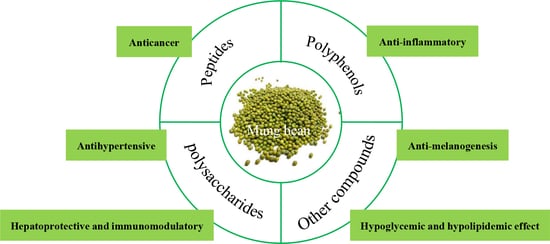
Mung Bean (Vigna radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits
Abstract
:1. Introduction
2. Polyphenolics
3. Polysaccharides
4. Peptides
5. Health Benefits
5.1. Hypoglycemic Properties
5.2. Hypolipidemic Properties
5.3. Hepatoprotective Properties
5.4. Antihypertensive Properties
5.5. Anticancer Properties
5.6. Immunomodulatory Activity
5.7. Anti-Melanogenesis Properties
5.8. Other Health Benefits
5.9. Health Benefits of the Mung Bean on Clinical Trials
6. Conclusions, Limitations of Current Knowledge, and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Mosa, K.A.; Ji, L.; Kage, U.; Dhokane, D.; Karre, S.; Madalageri, D.; Pathania, N. Metabolomics-assisted biotechnological interventions for developing plant-based functional foods and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2018, 58, 1791–1807. [Google Scholar] [CrossRef]
- Hou, D.; Chen, J.; Ren, X.; Wang, C.; Diao, X.; Hu, X.; Zhang, Y.; Shen, Q. A whole foxtail millet diet reduces blood pressure in subjects with mild hypertension. J. Cereal Sci. 2018, 84, 13–19. [Google Scholar] [CrossRef]
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef]
- Dahiya, P.K.; Linnemann, A.R.; Van Boekel, M.A.J.S.; Khetarpaul, N.; Grewal, R.B.; Nout, M.J.R. Mung bean: Technological and nutritional potential. Crit. Rev. Food Sci. Nutr. 2015, 55, 670–688. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.-Y.; Lui, W.-Y.; Wu, K.; Chan, C.-L.; Dai, S.-H.; Sui, Z.-Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Mubarak, A.E. Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 2005, 89, 489–495. [Google Scholar] [CrossRef]
- Yi-Shen, Z.; Shuai, S.; FitzGerald, R. Mung bean proteins and peptides: Nutritional, functional and bioactive properties. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Hussain, T.; Tontisirin, K.; Chaowanakarnkit, L. Protein digestibility of weaning foods prepared from rice-minced meat and rice-mungbean combination in infants using a short term nitrogen balance method. J. Nutr. Sci. Vitaminol. 1983, 29, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Rulli, M.C.; Santini, M. Human food vs. Animal feed debate. A thorough analysis of environmental footprints. Land Use Policy 2017, 67, 652–659. [Google Scholar] [CrossRef]
- Kumar Dahiya, P.; Nout, M.J.R.; A. van Boekel, M.; Khetarpaul, N.; Bala Grewal, R.; Linnemann, A. Nutritional characteristics of mung bean foods. Br. Food J. 2014, 116, 1031–1046. [Google Scholar] [CrossRef]
- Bazaz, R.; Baba, W.N.; Masoodi, F.A.; Yaqoob, S. Formulation and characterization of hypo allergic weaning foods containing potato and sprouted green gram. J. Food Meas. Charact. 2016, 10, 453–465. [Google Scholar] [CrossRef]
- Ali, S.; Singh, B.; Sharma, S. Response surface analysis and extrusion process optimisation of maize–mungbean-based instant weaning food. Int. J. Food Sci. Technol. 2016, 51, 2301–2312. [Google Scholar] [CrossRef]
- Nair, R.M.; Yang, R.-Y.; Easdown, W.J.; Thavarajah, D.; Thavarajah, P.; Hughes, J.d.A.; Keatinge, J. Biofortification of mungbean (Vigna radiata) as a whole food to enhance human health. J. Sci. Food Agric. 2013, 93, 1805–1813. [Google Scholar] [CrossRef]
- Sandberg, A.-S. Bioavailability of minerals in legumes. Br. J. Nutr. 2007, 88, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Barakoti, L.; Bains, K. Effect of household processing on the in vitro bioavailability of iron in mungbean (Vigna radiata). Food Nutr. Bull. 2007, 28, 18–22. [Google Scholar] [CrossRef]
- Tajoddin, M.D.; Shinde, M.; Junna, L. In vivo reduction the phytic acid content of mung bean (Phaseolus aureus L.) cultivars during germination. Am.-Eurasian J. Agric. Environ. Sci. 2011, 10, 127–132. [Google Scholar]
- Wang, X.; Yang, R.; Jin, X.; Chen, Z.; Zhou, Y.; Gu, Z. Effect of germination and incubation on zn, fe, and ca bioavailability values of soybeans (Glycine max L.) and mung beans (Vigna radiate L.). Food Sci. Biotechnol. 2015, 24, 1829–1835. [Google Scholar] [CrossRef]
- Liyanage, R.; Kiramage, C.; Visvanathan, R.; Jayathilake, C.; Weththasinghe, P.; Bangamuwage, R.; Chaminda Jayawardana, B.; Vidanarachchi, J. Hypolipidemic and hypoglycemic potential of raw, boiled, and sprouted mung beans (Vigna radiata L. Wilczek) in rats. J. Food Biochem. 2018, 42, e12457. [Google Scholar] [CrossRef]
- Ali, N.M.; Mohd, Y.H.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Long, K.; Koh, S.P.; Abdullah, M.P.; Alitheen, N.B. Anti-inflammatory and antinociceptive activities of untreated, germinated, and fermented mung bean aqueous extract. Evid.-Based Complement. Altern. Med. 2014, 2014, 350507. [Google Scholar] [CrossRef]
- Gupta, N.; Srivastava, N.; Bhagyawant, S.S. Vicilin—A major storage protein of mungbean exhibits antioxidative potential, antiproliferative effects and ace inhibitory activity. PLoS ONE 2018, 13, e0191265. [Google Scholar] [CrossRef]
- Chai, W.-M.; Wei, Q.-M.; Deng, W.-L.; Zheng, Y.-L.; Chen, X.-Y.; Huang, Q.; Ou-Yang, C.; Peng, Y.-Y. Anti-melanogenesis properties of condensed tannins from Vigna angularis seeds with potent antioxidant and DNA damage protection activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef]
- Xie, J.; Du, M.; Shen, M.; Wu, T.; Lin, L. Physico-chemical properties, antioxidant activities and angiotensin-i converting enzyme inhibitory of protein hydrolysates from mung bean (Vigna radiate). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.A.R.; Martins, M.D.C.d.C.e.; Farias, L.M.d.; Brito, A.K.d.S.; Lima, G.D.M.; Carvalho, V.B.L.d.; Pereira, C.F.d.C.; Conde Júnior, A.M.; Saldanha, T.; Arêas, J.A.G.; et al. Cholesterol-lowering and liver-protective effects of cooked and germinated mung beans (Vigna radiata L.). Nutrients 2018, 10, 821. [Google Scholar] [CrossRef]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Torregiani, E.; Vittori, S.; Sagratini, G. Analysis of 17 polyphenolic compounds in organic and conventional legumes by high-performance liquid chromatography-diode array detection (hplc-dad) and evaluation of their antioxidant activity. Int. J. Food Sci. Nutr. 2018, 69, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ketha, K.; Gudipati, M. Immunomodulatory activity of non starch polysaccharides isolated from green gram (Vigna radiata). Food Res. Int. 2018, 113, 269–276. [Google Scholar] [CrossRef]
- Sonklin, C.; Laohakunjit, N.; Kerdchoechuen, O. Assessment of antioxidant properties of membrane ultrafiltration peptides from mungbean meal protein hydrolysates. PeerJ 2018, 6, e5337. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, J.K.; Kim, S.G.; Kim, S.H.; Chun, T.; Imm, J.-Y. Comparative analyses of total phenols, flavonoids, saponins and antioxidant activity in yellow soy beans and mung beans. Int. J. Food Sci. Technol. 2011, 46, 2513–2519. [Google Scholar] [CrossRef]
- Shi, Z.; Yao, Y.; Zhu, Y.; Ren, G. Nutritional composition and antioxidant activity of twenty mung bean cultivars in china. Crop J. 2016, 4, 398–406. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, P.; Qin, F.; Zhou, Q.; Gao, B.; Huang, H.; Yang, H.; Shi, H.; Yu, L. Chemical composition and antioxidative and anti-inflammatory properties of ten commercial mung bean samples. LWT Food Sci. Technol. 2013, 54, 171–178. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, X.; Tian, J.; Liu, C.; Cheng, X.; Ren, G. Antioxidant and antidiabetic activities of black mung bean (Vigna radiata L.). J. Agric. Food Chem. 2013, 61, 8104–8109. [Google Scholar] [CrossRef] [PubMed]
- Meenu, M.; Kamboj, U.; Sharma, A.; Guha, P.; Mishra, S. Green method for determination of phenolic compounds in mung bean (Vigna radiata L.) based on near-infrared spectroscopy and chemometrics. Int. J. Food Sci. Technol. 2016, 51, 2520–2527. [Google Scholar] [CrossRef]
- Meenu, M.; Sharma, A.; Guha, P.; Mishra, S. A rapid high-performance liquid chromatography photodiode array detection method to determine phenolic compounds in mung bean (Vigna radiata L.). Int. J. Food Prop. 2016, 19, 2223–2237. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Q.; Wang, B.; Zhang, M.; Xu, Y.; Li, S.; Zhao, Y.; Yu, Z. Plasma pharmacokinetics, bioavailability, and tissue distribution of four c-glycosyl flavones from mung bean (Vigna radiata L.) seed extracts in rat by ultrahigh-performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2017, 65, 5570–5580. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Cao, D.; Li, H.; Yi, J.; Zhang, J.; Che, H.; Cao, J.; Yang, L.; Zhu, C.; Jiang, W. Antioxidant properties of the mung bean flavonoids on alleviating heat stress. PLoS ONE 2011, 6, e21071. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, Z.; Cheng, K.-W.; Shan, F.; Ren, G.-X.; Chen, F.; Wang, M. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008, 106, 475–481. [Google Scholar] [CrossRef]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef]
- Li, H.; Cao, D.; Yi, J.; Cao, J.; Jiang, W. Identification of the flavonoids in mungbean (Phaseolus radiatus L.) soup and their antioxidant activities. Food Chem. 2012, 135, 2942–2946. [Google Scholar] [CrossRef]
- Soccio, M.; Laus, M.N.; Alfarano, M.; Dalfino, G.; Panunzio, M.F.; Pastore, D. Antioxidant/oxidant balance as a novel approach to evaluate the effect on serum of long-term intake of plant antioxidant-rich foods. J. Funct. Foods 2018, 40, 778–784. [Google Scholar] [CrossRef]
- Soccio, M.; Laus, M.N.; Flagella, Z.; Pastore, D. Assessment of antioxidant capacity and putative healthy effects of natural plant products using soybean lipoxygenase-based methods. An overview. Molecules 2018, 23, 3244. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Prokudina, E.A.; Havlíček, L.; Al-Maharik, N.; Lapčík, O.; Strnad, M.; Gruz, J. Rapid uplc–esi–ms/ms method for the analysis of isoflavonoids and other phenylpropanoids. J. Food Compos. Anal. 2012, 26, 36–42. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.; Tang, K.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef]
- Mohd Ali, N.; Mohd Yusof, H.; Long, K.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Koh, S.P.; Abdullah, M.P.; Alitheen, N.B. Antioxidant and hepatoprotective effect of aqueous extract of germinated and fermented mung bean on ethanol-mediated liver damage. BioMed Res. Int. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Yeap, S.K.; Beh, B.K.; Ho, W.Y.; Mohd Yusof, H.; Mohamad, N.E.; Ali, N.M.; Jaganath, I.B.; Alitheen, N.B.; Koh, S.P.; Long, K. In vivo antioxidant and hypolipidemic effects of fermented mung bean on hypercholesterolemic mice. Evid.-Based Complement. Altern. Med. eCAM 2015, 2015, 508029. [Google Scholar] [CrossRef]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Lai, F.; Wen, Q.; Li, L.; Wu, H.; Li, X. Antioxidant activities of water-soluble polysaccharide extracted from mung bean (Vigna radiata L.) hull with ultrasonic assisted treatment. Carbohydr. Polym. 2010, 81, 323–329. [Google Scholar] [CrossRef]
- Zhong, K.; Lin, W.; Wang, Q.; Zhou, S. Extraction and radicals scavenging activity of polysaccharides with microwave extraction from mung bean hulls. Int. J. Biol. Macromol. 2012, 51, 612–617. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.; Ren, G. Immunoregulatory activities of polysaccharides from mung bean. Carbohydr. Polym. 2016, 139, 61–66. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.; Ren, G. Antioxidant and immunoregulatory activity of alkali-extractable polysaccharides from mung bean. Int. J. Biol. Macromol. 2016, 84, 289–294. [Google Scholar] [CrossRef]
- Ketha, K.; Gudipati, M. Purification, structural characterization of an arabinogalactan from green gram (Vigna radiata) and its role in macrophage activation. J. Funct. Foods 2018, 50, 127–136. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Wongekalak, L.-o.; Sakulsom, P.; Jirasripongpun, K.; Hongsprabhas, P. Potential use of antioxidative mungbean protein hydrolysate as an anticancer asiatic acid carrier. Food Res. Int. 2011, 44, 812–817. [Google Scholar] [CrossRef]
- Li, G.H.; Le, G.W.; Liu, H.; Shi, Y.H. Mung-bean protein hydrolysates obtained with alcalase exhibit angiotensin i-converting enzyme inhibitory activity. Food Sci. Technol. Int. 2005, 11, 281–287. [Google Scholar] [CrossRef]
- Li, G.-H.; Wan, J.-Z.; Le, G.-W.; Shi, Y.-H. Novel angiotensin i-converting enzyme inhibitory peptides isolated from alcalase hydrolysate of mung bean protein. J. Pept. Sci. 2006, 12, 509–514. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, Y.; Chang, J.; Wang, X.; Zhao, Y.; Yu, Z. Bioactives from stems and leaves of mung beans (Vigna radiata L.). J. Funct. Foods 2016, 25, 314–322. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, K. Mung beans processed by solid-state bioconversion improves phenolic content and functionality relevant for diabetes and ulcer management. Innov. Food Sci. Emerg. Technol. 2007, 8, 197–204. [Google Scholar] [CrossRef]
- Jang, Y.-H.; Kang, M.-J.; Choe, E.-O.; Shin, M.; Kim, J.-I. Mung bean coat ameliorates hyperglycemia and the antioxidant status in type 2 diabetic db/db mice. Food Sci. Biotechnol. 2014, 23, 247–252. [Google Scholar] [CrossRef]
- Inhae, K.; Seojin, C.; Joung, H.T.; Munji, C.; Hae-Ri, W.; Won, L.B.; Myoungsook, L. Effects of mung bean (Vigna radiata L.) ethanol extracts decrease proinflammatory cytokine-induced lipogenesis in the kk-ay diabese mouse model. J. Med. Food 2015, 18, 841–849. [Google Scholar]
- Joghatai, M.; Barari, L.; Mousavie Anijdan, S.H.; Elmi, M.M. The evaluation of radio-sensitivity of mung bean proteins aqueous extract on mcf-7, hela and fibroblast cell line. Int. J. Radiat. Biol. 2018, 94, 478–487. [Google Scholar] [CrossRef]
- Wang, S.; Lin, J.; Ye, M.; Ng, T.B.; Rao, P.; Ye, X. Isolation and characterization of a novel mung bean protease inhibitor with antipathogenic and anti-proliferative activities. Peptides 2006, 27, 3129–3136. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef]
- Kim, D.-K.; Jeong, S.C.; Gorinstein, S.; Chon, S.-U. Total polyphenols, antioxidant and antiproliferative activities of different extracts in mungbean seeds and sprouts. Plant Foods Hum. Nutr. 2012, 67, 71–75. [Google Scholar] [CrossRef]
- Hafidh, R.R.; Abdulamir, A.S.; Bakar, F.A.; Jalilian, F.A.; Abas, F.; Sekawi, Z. Novel molecular, cytotoxical, and immunological study on promising and selective anticancer activity of mung bean sprouts. BMC Complement. Altern. Med. 2012, 12, 208. [Google Scholar] [CrossRef]
- Dai, Z.; Su, D.; Zhang, Y.; Sun, Y.; Hu, B.; Ye, H.; Jabbar, S.; Zeng, X. Immunomodulatory activity in vitro and in vivo of verbascose from mung beans (Phaseolus aureus). J. Agric. Food Chem. 2014, 62, 10727–10735. [Google Scholar] [CrossRef]
- Ali, N.M.; Yeap, S.-K.; Yusof, H.M.; Beh, B.-K.; Ho, W.-Y.; Koh, S.-P.; Abdullah, M.P.; Alitheen, N.B.; Long, K. Comparison of free amino acids, antioxidants, soluble phenolic acids, cytotoxicity and immunomodulation of fermented mung bean and soybean. J. Sci. Food Agric. 2016, 96, 1648–1658. [Google Scholar] [CrossRef]
- Lee, S.J.; Bae, J.; Kim, S.; Jeong, S.; Choi, C.-Y.; Choi, S.-P.; Kim, H.-S.; Jung, W.-W.; Imm, J.-Y.; Kim, S.H.; et al. Saponins from soy bean and mung bean inhibit the antigen specific activation of helper t cells by blocking cell cycle progression. Biotechnol. Lett. 2013, 35, 165–173. [Google Scholar] [CrossRef]
- Zhu, S.; Li, W.; Li, J.; Jundoria, A.; Sama, A.E.; Wang, H. It is not just folklore: The aqueous extract of mung bean coat is protective against sepsis. Evid.-Based Complement. Altern. Med. 2012, 2012, 498467. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, J.H.; Lee, H.-H.; Lee, S.; Kim, S.H.; Chun, T.; Imm, J.-Y. Effect of mung bean ethanol extract on pro-inflammtory cytokines in lps stimulated macrophages. Food Sci. Biotechnol. 2011, 20, 519–524. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. Mushroom tyrosinase inhibitors from mung bean (Vigna radiatae L.) extracts. Int. J. Food Sci. Nutr. 2012, 63, 358–361. [Google Scholar] [CrossRef]
- Jeong, Y.M.; Ha, J.H.; Noh, G.Y.; Park, S.N. Inhibitory effects of mung bean (Vigna radiata L.) seed and sprout extracts on melanogenesis. Food Sci. Biotechnol. 2016, 25, 567–573. [Google Scholar] [CrossRef]
- Chai, W.-M.; Ou-Yang, C.; Huang, Q.; Lin, M.-Z.; Wang, Y.-X.; Xu, K.-L.; Huang, W.-Y.; Pang, D.-D. Antityrosinase and antioxidant properties of mung bean seed proanthocyanidins: Novel insights into the inhibitory mechanism. Food Chem. 2018, 260, 27–36. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. Biological potential of sixteen legumes in china. Int. J. Mol. Sci. 2011, 12, 7048–7058. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Imran, M.; Zahoor, T.; Ahmad, R.S.; Arshad, M.U. Biochemical perspectives of xylitol extracted from indigenous agricultural by-product mung bean (Vigna radiata) hulls in a rat model. J. Sci. Food Agric. 2014, 94, 969–974. [Google Scholar] [CrossRef]
- Yeap, S.K.; Mohd Ali, N.; Mohd Yusof, H.; Alitheen, N.B.; Beh, B.K.; Ho, W.Y.; Koh, S.P.; Long, K. Antihyperglycemic effects of fermented and nonfermented mung bean extracts on alloxan-induced-diabetic mice. J. Biomed. Biotechnol. 2012, 2012, 285430. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, F.; Wang, M.; Wang, J.; Ren, G. Antidiabetic activity of mung bean extracts in diabetic kk-ay mice. J. Agric. Food Chem. 2008, 56, 8869–8873. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Koyama, M.; Ishida, R.; Kitahara, T.; Nakajima, T.; Aoyama, T. Characterization of bioactive agents in five types of marketed sprouts and comparison of their antihypertensive, antihyperlipidemic, and antidiabetic effects in fructose-loaded shrs. J. Food Sci. Technol. 2016, 53, 581–590. [Google Scholar] [CrossRef]
- Yao, Y.; Hao, L.; Shi, Z.; Wang, L.; Cheng, X.; Wang, S.; Ren, G. Mung bean decreases plasma cholesterol by up-regulation of cyp7a1. Plant Foods Hum. Nutr. 2014, 69, 134–136. [Google Scholar] [CrossRef]
- Asrullah, M.; Lestari, L.A.; Helmyati, S.; Farmawati, A. The effect of mung bean sprouts (Phaseolus radiatus L.) to lipid profile of male sprague-dawley rats fed with high-fat diet. AIP Conf. Proc. 2016, 1755, 140001. [Google Scholar]
- Nakatani, A.; Li, X.; Miyamoto, J.; Igarashi, M.; Watanabe, H.; Sutou, A.; Watanabe, K.; Motoyama, T.; Tachibana, N.; Kohno, M.; et al. Dietary mung bean protein reduces high-fat diet-induced weight gain by modulating host bile acid metabolism in a gut microbiota-dependent manner. Biochem. Biophys. Res. Commun. 2018, 501, 955–961. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.; Ren, G. Mung bean protein increases plasma cholesterol by up-regulation of hepatic hmg-coa reductase, and cyp7a1 in mrna levels. J. Food Nutr. Res. 2014, 2, 770–775. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Balakrishnan, A.; Chinnasamy, T. Protective role of germinated mung bean against progression of non-alcoholic steatohepatitis in rats: A dietary therapy to improve fatty liver health. J. Food Biochem. 2018, 42, e12542. [Google Scholar] [CrossRef]
- Watanabe, H.; Inaba, Y.; Inoue, H.; Kimura, K.; Kaneko, S.; Asahara, S.-I.; Kido, Y.; Matsumoto, M.; Kohno, M.; Tachibana, N.; et al. Dietary mung bean protein reduces hepatic steatosis, fibrosis, and inflammation in male mice with diet-induced, nonalcoholic fatty liver disease. J. Nutr. 2016, 147, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xiao, H.Y.; Gao, E.Z.; Xiao, N.L.; Li, J.S.; Hua, L.L.; Pei, W.; Yun, L.Z.; Zhi, G.Y. Hepatoprotective effect of active constituents isolated from mung beans (Phaseolus radiatus L.) in an alcohol-induced liver injury mouse model. J. Food Biochem. 2015, 38, 453–459. [Google Scholar] [CrossRef]
- Wu, S.J.; Wang, J.S.; Lin, C.C.; Chang, C.H. Evaluation of hepatoprotective activity of legumes. Phytomedicine 2001, 8, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-H.; Shi, Y.-H.; Liu, H.; Le, G.-W. Antihypertensive effect of alcalase generated mung bean protein hydrolysates in spontaneously hypertensive rats. Eur. Food Res. Technol. 2006, 222, 733–736. [Google Scholar] [CrossRef]
- Hsu, G.; Lu, Y.; Chang, S.; Hsu, S. Antihypertensive effect of mung bean sprout extract in spontaneously hypertensive rats. J. Food Biochem. 2011, 35, 278–288. [Google Scholar] [CrossRef]
- Yeap, S.K.; Mohd Yusof, H.; Mohamad, N.E.; Beh, B.K.; Ho, W.Y.; Ali, N.M.; Alitheen, N.B.; Koh, S.P.; Long, K. In vivo immunomodulation and lipid peroxidation activities contributed to chemoprevention effects of fermented mung bean against breast cancer. Evid.-Based Complement. Altern. Med. 2013, 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Venkateshwarlu, E.; Reddy, K.P.; Dilip, D. Potential of Vigna radiata (L.) sprouts in the management of inflammation and arthritis in rats: Possible biochemical alterations. Indian J. Exp. Biol. 2016, 54, 37. [Google Scholar]
- Westman, E.C.; Yancy, W.S.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 2008, 5, 36. [Google Scholar] [CrossRef]
- Noakes, M.; Brinkworth, G.D.; Luscombe-Marsh, N.D.; Tay, J.; Thompson, C.H.; Wittert, G.A.; Buckley, J.D.; Yancy, W.S., Jr. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar]
- Lerer-Metzger, M.; Rizkallal, S.W.; Luo, J.; Champ, M.; Kabir, M.; Bruzzo, F.; Bornet, F.; Slama, G. Effects of long-term low-glycaemic index starchy food on plasma glucose and lipid concentrations and adipose tissue cellularity in normal and diabetic rats. Br. J. Nutr. 1996, 75, 723–732. [Google Scholar] [CrossRef]
- van Amelsvoort, J.M.; Weststrate, J.A. Amylose-amylopectin ratio in a meal affects postprandial variables in male volunteers. Am. J. Clin. Nutr. 1992, 55, 712–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, W.; Zhang, A.Z.; Jiang, N.; Zhu, S.; Zhao, F.F.; Wu, Q.; Liu, W.; Wang, L.X.; Cai, P.; Wang, F.M.; et al. Effects of different amylose to amylopectin ratios on serum indices related to glucose metabolism and glucose transporter expression in fattening lambs. Animal Feed Sci. Technol. 2015, 202, 106–111. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Kabir, M.; Rizkalla, S.W.; Champ, M.; Luo, J.; Boillot, J.; Bruzzo, F.; Slama, G. Dietary amylose-amylopectin starch content affects glucose and lipid metabolism in adipocytes of normal and diabetic rats. J. Nutr. 1998, 128, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The glycemic indexphysiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Cederbaum, A.I. Alcohol and oxidative liver injury. Hepatology 2006, 43, S63–S74. [Google Scholar] [CrossRef]
- Stickel, F.; Kessebohm, K.; Weimann, R.; Seitz, H.K. Review of liver injury associated with dietary supplements. Liver Int. 2011, 31, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Golabi, P.; Bush, H.; Younossi, Z.M. Treatment strategies for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin. Liver Dis. 2017, 21, 739–753. [Google Scholar] [CrossRef]
- Stagos, D.; Amoutzias, G.D.; Matakos, A.; Spyrou, A.; Tsatsakis, A.M.; Kouretas, D. Chemoprevention of liver cancer by plant polyphenols. Food Chem. Toxicol. 2012, 50, 2155–2170. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Zhang, Q.; Shen, Q. Recent research in antihypertensive activity of food protein-derived hydrolyzates and peptides. Crit. Rev. Food Sci. Nutr. 2016, 56, 760–787. [Google Scholar] [CrossRef] [PubMed]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T.; et al. American cancer society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef]
- Thompson, M.D.; Brick, M.A.; McGinley, J.N.; Thompson, H.J. Chemical composition and mammary cancer inhibitory activity of dry bean. Crop Sci. 2009, 49, 179–186. [Google Scholar] [CrossRef]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100, 437S–442S. [Google Scholar] [CrossRef] [Green Version]
- Galli, C.; Calder, P.C. Effects of fat and fatty acid intake on inflammatory and immune responses: A critical review. Ann. Nutr. Metab. 2009, 55, 123–139. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343. [Google Scholar] [CrossRef]
- Hoebe, K.; Janssen, E.; Beutler, B. The interface between innate and adaptive immunity. Nat. Immunol. 2004, 5, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Varin, A.; Gordon, S. Alternative activation of macrophages: Immune function and cellular biology. Immunobiology 2009, 214, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2009, 20, 34. [Google Scholar] [CrossRef]
- Vos, A.P.; M’Rabet, L.; Stahl, B.; Boehm, G.; Garssen, J. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit. Rev.™ Immunol. 2007, 27, 97–140. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Abbas, A.K.; Murphy, K.M.; Sher, A. Functional diversity of helper t lymphocytes. Nature 1996, 383, 787–793. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Gao, E.; Yu, X.; Liu, T.; Li, H.; Wang, P.; Wei, Y.; Zhao, Y.; Yu, Z. Comparative study on effects of single and multiple oral administration of mungbean (Phaseolus radiatus L.) seed extract on the pharmacokinetics of aconitine by uhplc-ms. Biomed. Chromatogr. 2014, 28, 1313–1319. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, R.; Wang, C.; Hou, L. Mung bean (Phaseolus radiatus L.) polyphenol extract attenuates aluminum-induced cardiotoxicity through an ros-triggered ca2+/jnk/nf-κb signaling pathway in rats. Food Funct. 2017, 8, 851–859. [Google Scholar] [CrossRef]
- Bai, Y.; Chang, J.; Xu, Y.; Cheng, D.; Liu, H.; Zhao, Y.; Yu, Z. Antioxidant and myocardial preservation activities of natural phytochemicals from mung bean (Vigna radiata L.) seeds. J. Agric. Food Chem. 2016, 64, 4648–4655. [Google Scholar] [CrossRef]
- Bornet, F.R.; Fontvieille, A.M.; Rizkalla, S.; Colonna, P.; Blayo, A.; Mercier, C.; Slama, G. Insulin and glycemic responses in healthy humans to native starches processed in different ways: Correlation with in vitro alpha-amylase hydrolysis. Am. J. Clin. Nutr. 1989, 50, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Lang, V.; Bornet, F.R.; Vaugelade, P.; van Ypersele de Strihou, M.; Luo, J.; Pacher, N.; Rossi, F.; La Droitte, P.; Duée, P.-H.; Slama, G. Euglycemic hyperinsulinemic clamp to assess posthepatic glucose appearance after carbohydrate loading. 2. Evaluation of corn and mung bean starches in healthy men. Am. J. Clin. Nutr. 1999, 69, 1183–1188. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M.; Ohkawara, T.; Sato, Y.; Satoh, H.; Takahashi, Y.; Hajika, M.; Nishihira, J. Improvement of triglyceride levels through the intake of enriched-β-conglycinin soybean (nanahomare) revealed in a randomized, double-blind, placebo-controlled study. Nutrients 2016, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Sugano, H.; Shigihara, Y.; Shiraishi, Y.; Motoyama, T. Improvement of glucose and lipid metabolism via mung bean protein consumption: Clinical trials of glucodia™ isolated mung bean protein in the USA and canada. J. Nutr. Sci. 2018, 7, e2. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Motoyama, T.; Shigihara, Y.; Sakamoto, M.; Sugano, H. Improvement of glucose metabolism via mung bean protein consumption: A clinical trial of glucodia tm isolated mung bean protein in japan. Funct. Foods Health Dis. 2017, 7, 115–134. [Google Scholar]
- Bhan, M.K.; Ghai, O.P.; Khoshoo, V.; Vasudev, A.S.; Bhatnagar, S.; Arora, N.K.; Rashmi; Stintzing, G. Efficacy of mung bean (lentil) and pop rice based rehydration solutions in comparison with the standard glucose electrolyte solution. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 392–399. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [Green Version]



| Class | Subclass | Compound | Content | References |
|---|---|---|---|---|
| Flavonoids | Anthocyanins | Cyanidin-3-glucoside | 256.32–476.53 μg/g | [35] |
| Peonidin-3-glucoside | 5.43- 8.42 μg/g | [35] | ||
| Pelargonidin-3,6-malonylglucoside | 8.53–12.49 μg/g | [35] | ||
| Pelargonidin-3-glucoside | 147.62–350.71 μg/g | [35] | ||
| Flavonols | Quercetin | 0.17–16.2 mg/100 g | [36,37,38] | |
| Myricetin | 0.03–4.26 mg/100 g | [36,37] | ||
| Kaempferol | 0.07–6.13 mg/100 g | [36,37] | ||
| Flavanols | Catechin | 4.39–35.36 mg/100 g | [36,37] | |
| Flavones | Vitexin | 17.04–62.37 mg/100 g | [36,37] | |
| Isovitexin | 22.63–73.64 mg/100 g | [36,37] | ||
| Isovitexin-6″-O-α-L-glucoside | 1.70 mg/g | [39] | ||
| Luteolin | 0.36 mg/100 g | [38] | ||
| Isoflavonoids | Dulcinoside | 0.13 mg/g | [39] | |
| Phenolic acids | Hydroxycinnamic acid | p-Coumaric acid | 8.17–38.34 mg/100 g | [36,37] |
| Caffeic acid | 1.37–38.72 mg/100 g | [36,37] | ||
| t-ferulic acid | 8.02–54.77 mg/100 g | [36,37] | ||
| Chlorogenic acid | 0–26.55 mg/100 g | [36] | ||
| Sinapic acid | 22.46 mg/100 g | [38] | ||
| Hydroxybenzoic acid | Gallic acid | 1.32–9.47 mg/100 g | [36,38] | |
| Syringic | 0–220.29 μg/g | [35] | ||
| Gentisic | 25.39–138.45 μg/g | [35] |
| Compound Name | Molecular Weight (kDa) | Monosaccharide Composition (Molar Ratio, %) | Bioactivities | Reference |
|---|---|---|---|---|
| MP1 | 83 | Fuc:Ara:Xyl:Man:Gal:Glu = 8.3:2.2:67.2:20.1:2.3 | Antioxidant activities | [55] |
| MP2 | 45 | Rha:Fuc:fucose:Ara:Xyl:Man:Gal:Glu = 31.8:3.5:16.7:4.6:11.7:29.1:2.5 | Antioxidant activities | [55] |
| MEMP-1 | Nd 1 | Ara:Man:Gal = 0.12:1.0:0.5 | Radicals scavenging activity | [56] |
| MEMP-2 | Nd | Rha:Ara:Man:Gal = 1:0.3:0.3:0.6 | Radicals scavenging activity | [56] |
| MWP-1′ | 68.4 | Rha:Ara:Man:Galin = 0.4:2.6:5.3:0.7 | Immunoregulatory activities | [57] |
| MWP-2′ | 52.4 | Ara:Man:Gal:Glc = 0.5:1.4:2.1:0.4 | Immunoregulatory activities | [57] |
| MAP-1 | 94.2 | Rha:Ara:Glu:Gal:GalA = 1.1:0.4:0.7:0.5:0.3 | Antioxidant and immunoregulatory activity | [58] |
| MAP-2 | 60.4 | Xyl:Rha:Gal:Glu:GalA = 0.4:1.4:1.6:0.5:0.2 | Antioxidant and immunoregulatory activity | [58] |
| WSP | 20–300 | Ara:Man:Gal:Glu = 3.5:12:66:18.5 | Nd | [28] |
| HWSP | 15–150 | Rha:Ara:Xyl:Man:Gal:Glu = 2.5:34:5:8:33.5:17 | Immunomodulatory activity | [28] |
| Pectins | 40–1200 | Rha:Ara:Xyl:Gal:Glu = 2.6:46:8:26.4:17 | Immunomodulatory activity | [28] |
| Hemicellulose A | 15–350 | Ara:Xyl:Man:Gal:Glu = 8.5:23:2:9:57.5 | Nd | [28] |
| Hemicellulose B | 100–1800 | Rha:Ara:Xyl:Gal:Glu = 2.5:43:7:28:19.5 | Immunomodulatory activity | [28] |
| Arabinogalactan | 1200 | Rha:Ara:Xyl:Gal:Glu = 5:60:2:32:1 | Macrophage activation | [59] |
| Health Benefits | Model | Type of Extract/Constituents | Dose/Reaction System | Experimental Outcome | Reference |
|---|---|---|---|---|---|
| Hypoglycemic properties | Biochemical tests | Lignans and flavonoids | 20 μL/1650 μL | Inhibited the activity of α-glucosidase | [64] |
| Biochemical tests | Vitexin and isovitexin | 500 ppm, 100 μm | Inhibited the formation of advanced glycation end products | [44] | |
| Biochemical tests | Phenolic compounds | 50 μL/200 μL, 1 mL/3 mL | Inhibited the activity of α-glucosidase and the formation of advanced glycation end products | [35] | |
| Biochemical tests | Aqueous extracts of raw, boiled, and sprouted mung bean | 20 μL/220 μL, 40 μL/260 μL | Inhibited the activity of α-glucosidase and α-amylase | [21] | |
| Biochemical tests | Aqueous extract of bioprocessed mung bean | 800 μL/6 mL | Inhibited the activity of α-Amylase | [65] | |
| Biochemical tests | Ethanolic extracts of whole mung bean, cotyledon, and hull | 0.1 mL/1 mL | Inhibited the activity of aldose reductase | [45] | |
| Biochemical tests | Ethanolic extract of mung bean seed coat | 5 mg/mL | Inhibited the activity of α-glucosidase | [66] | |
| Hypolipidemic properties | 3T3-L1 preadipocytes | Vitexin and isovitexin | 25, 50 and 100 μM | Decreased fat accumulation Lowered inflammatory cytokines, IL-6 and MCP-1 | [67] |
| Antihypertensive properties | Biochemical tests | Mung bean protein hydrolysates | 5, 7.5, 10, 12.5, 15, 20 and 25 μg/mL | Exhibited ACE-I inhibitory activity | [25] |
| Biochemical tests | Vicilin protein (storage protein of mung bean) hydrolysate | 0.2–1.0 mg/mL | Exhibited ACE-I inhibitory activity | [23] | |
| Biochemical tests | Mung bean protein hydrolysate | 100 μg/mL | Exhibited ACE-I inhibitory activity | [63] | |
| Biochemical tests | Mung bean protein hydrolysate | 10 mg protein/mL | Exhibited ACE-I inhibitory activity | [62] | |
| Anticancer properties | Human breast adenocarcinoma cells (MCF-7), human cervical cancer cells (Hela) | Proteins isolated from mung bean aqueous extract | 62.5, 125, 250, 500 and 1000 μg/mL | Exhibited the anti-proliferation activities | [68] |
| Human breast adenocarcinoma cells (MCF-7 and MDA-MB-231) | vicilin protein (storage protein of mung bean) hydrolysate | 10, 25, 50, 75 and 100 mg/mL | Exhibited the anti-proliferation activities | [23] | |
| Human hepatoma cells (Bel-7402) | Mungoin- a novel mung bean protease inhibitor | 10, 50, 100 and 200 μM | Exhibited the anti-proliferation activities | [69] | |
| Digestive system cancer cells (CAL27, AGS, HepG2, SW480 and Caco-2), prostate cancer cells (DU145), ovary cancer cells (SK-OV-3), breast cancer cells (MCF-7), and leukemia cells (HL-60) | Phenolics | 0.125, 0.25, 0.5, 1, 2 and 5 mg/mL | Exhibited the anti-proliferation activities | [70] | |
| Human pulmonary carcinoma cell, human gastric carcinoma cells (SNU-601) | Aqueous, ethyl acetate, methanol, n-hexane, n-butanol extracts of mung bean seeds and sprouts | Nd 1 | Exhibited the anti-proliferation activities | [71] | |
| Cervix adenocarcinoma cells (HeLa; ATCC CCL-2), hepatocellular carcinoma (HepG2; ATCC HB-8065) | Methanol Extracts of mung bean sprouts | 9.37 to 300 mg/mL, 10.25 to 164 mg/mL, 3.12 to 100 mg/mL, 0.31 to 10 mg/mL | Increased levels of anticancer cytokine (TNF-α and IFN-β) Induced IFN-γ and inhibited IL-4 production Induced apoptosis in HeLa and HepG2 cells Induced cell cycle arrest in HeLa Induced cdk-inhibitor proteins (p21, p53, and p27) in HeLa cells Induced only p53 in HepG2 cells | [72] | |
| Immunomodulatory activity | Murine macrophage RAW264.7 cells | Verbascose | 25, 50, 100, 200, and 400 μg/mL | Enhanced the ability of devouring neutral red of peritoneal macrophages Promoted the release of NO and immune reactive molecules, IL-6, IL-1β, IFN-α, and IFN-γ | [73] |
| Murine macrophage RAW264.7 cells | Arabinogalactan | 10, 50, 100, and 200 μg/mL | Induced the release of NO, TNF-α, IL-6, and IL-1β Increased phagocytic capability of macrophages | [59] | |
| Male Balb/c mice splenocyte (8–10 week-old) | Aqueous extract of fermented mung bean | 2.3 mg/mL | Enhanced splenocyte proliferation Increased serum IL-2 and ŽIFN-γ concentrations | [74] | |
| Murine macrophage RAW264.7 cells | Water-extractable polysaccharides from mung beans | 50, 100 and 200 μg/mL | Stimulate the production of NO, TNF-α and IL-6 | [57] | |
| Murine macrophage RAW264.7 cells | Alkali-extractable polysaccharides from mung beans | 50, 100 and 200 μg/mL | Stimulated the production of NO, TNF-α and IL-6 | [58] | |
| Male Wistar splenocytes (8 week-old), murine macrophage RAW264.7 cells | Water soluble (cold and hot water, 55 °C), EDTA soluble (0.5%, Pectins), alkali-soluble (10%, Hemicellulose A and B) polysaccharides isolated from mung beans | 0.1–100 μg/mL, 50–1000 μg/mL | Enhanced splenocyte proliferation Increased the production of NO | [28] | |
| T helper cells (transgenic OT-II mice) | Saponins | 50 and 100 μg/mL | Inhibited Th cell proliferation | [75] | |
| Murine macrophage RAW264.7 cells | Vitexin and isovitexin | 100 μg/mL | Inhibited the expression of IL-1β, IL-6, and COX-2 mRNA | [34] | |
| Murine macrophage RAW264.7 cells | Aqueous extracts of untreated, germinated, and fermented mung beans | 2.5 and 5 mg/mL | Decreased level of NO | [22] | |
| Murine macrophage RAW264.7 cells and human monocyte U-937 cells | Aqueous extract of mung bean seed coat | 0.1, 0.2, 0.8, 4, 8, and 15 mg/L | Reduced both intra- and extracellular HMGB1 levels in endotoxin-stimulated macrophages Stimulated HMGB1 protein aggregation Facilitated the formation of microtubule-associated protein-1-light-chain-3-(LC3-) and the production of LC3-II | [76] | |
| Biochemical tests | Ethanolic extracts of whole mung bean, cotyledon, and hull | 1 mL/5 mL | Inhibited the activity of protease | [45] | |
| Macrophages cells (J774) | Ethanolic extract of mung bean | 3.7 mg/mL | Decreased the mRNA expression of IL-1β, IL-6, IL-12β, TNF-α, and iNOS | [77] | |
| Anti-melanogenesis properties | Biochemical tests | Vitexin and isovitexin | 10 and 15 μM | Inhibited tyrosinase activity | [78] |
| Mouse melanoma cells (B16F1) | Vitexin and isovitexin | 10–250 μg/mL | Inhibited melanogenesis | [79] | |
| Mouse melanoma cells (B16) | Tannins | 50, 100, 200, and 400 μg/mL | Inhibited cell proliferation, cellular tyrosinase activity, and melanogenesis | [24] | |
| Biochemical tests | Antityrosinase | 20, 40, 60, 80, 120, 160, and 200 μg/mL | Inhibited the monophenolase and diphenolase activities | [80] | |
| Biochemical tests | Ethanolic extract of mung beans | 15 mg/mL | Inhibited tyrosinase activity | [81] | |
| Biochemical tests | Aqueous, ethyl acetate, methanol, n-hexane, n-butanol extracts of mung bean seeds and sprouts | Nd | Inhibited tyrosinase activity | [71] |
| Health Benefits | Model | Dose and Duration | Experimental Outcome | Reference |
|---|---|---|---|---|
| Hypoglycemic properties | Wistar rats in a cholesterol-enriched diet | Raw, boiled, and sprouted mung beans (30%) in diet supplementation for 5 weeks | ↓ Blood glucose, insulin, TG, non-HDL-C, HDL-C ↑ Liver weight, cecal weight, fecal matter weight | [21] |
| STZ-induced diabetes rats | Fermented mung bean seed coat (100 and 200 g/kg) in diet supplementation for 21 days | ↓ Feed intake, body weight, ↓ serum glucose, TC, TG ↑ Liver weight, cecum weight | [82] | |
| Alloxan-induced diabetes mice | Aqueous extracts of fermented and nonfermented mung bean (200 and 1000 mg/kg) for 10 days | ↓ Blood glucose, TG, LDL, NO level | [83] | |
| Diabetic db/db mice | Ethanolic extract of mung bean seed coat (1%) in diet supplementation for 7 weeks | ↓ Fasting serum glucose, blood glycated hemoglobin, HOMA-IR ↓ Lipid peroxides ↑ SOD activity, CAT activity, GSH-Px activity | [66] | |
| Diabetic KK-Ay mice | Ethanolic extracts of mung bean sprout and mung bean seed coat (2 and 3 g/kg) for 5 weeks | ↓ Body weight, blood glucose level, C-peptide level, glucagon level, TC | [84] | |
| Hypolipidemic properties | Fructose-loaded spontaneously hypertensive rats | Mung bean sprouts (30%) in diet supplementation for 46 days | ↓ TG, TC ↓ Heat rate | [85] |
| Hamsters in a cholesterol-enriched diet | Mung bean (1% and 2%) in diet supplementation for 6 weeks | ↓ Plasma TC, TG, non-HDL-C, non-HDL-C/HDL-C, and TC/HDL-C, liver cholesterol ↑ Coprostanol, total neutral sterol, deoxycholic acid, chenodeoxycholic acid, ursocholic acid, total acidic sterol, cholesterol intake, total sterol excretion ↓ Apparent cholesterol absorption ↑ The protein level of CYP7A1 | [86] | |
| High-fat-diet-induced rats | The juice of mung bean sprout (0.67 and 1.34 mg/200 g) for 28 days | ↓ TC, TG, LDL ↑ HDL | [87] | |
| Balb/c mice in a cholesterol-enriched diet | Aqueous extracts of fermented and nonfermented mung beans (200 and 1000 mg/kg) for 2 weeks | ↓TC, TG, LDL, ALT, ALP ↓ MDA, FRAP, NO ↑ HDL, SOD | [53] | |
| High-fat diet-induced male C57BL/6 mice | Mung bean protein isolate in diet supplementation (26.35%) for 4 weeks | ↓ body weight, epididymal, perirenal, and subcutaneous adipose weights, hepatic triglyceride ↑ Glucagon-like peptide-1, secondary/primary bile acid ratio, phylum Bacteroidetes ↓ Abundance of the Firmicutes | [88] | |
| Hamsters in a cholesterol-enriched diet | Mung bean protein isolate in diet supplementation (1% and 2%) for 6 weeks | ↓ TC, TG, non-HDL-C, non-HDL-C/HDL-C, and TC/HDL-C ↑ Sterol excretion ↓ The cholesterol absorption ↑ The production of mRNA3-hydroxy-3-methyl glutaryl coenzyme A reductase, CYP7A1 | [89] | |
| Hepatoprotective | Hamsters in a casein hypercholesterolemic diet | Cooked and germinated mung beans (as 22.1% protein source) in diet supplementation for 28 days | ↓ Relative liver weight, non-HDL-C, AST, ALT ↑ Fecal cholesterol | [26] |
| Methionine and choline-deficient diet induced steatohepatitis rats | Germinated mung bean power (500 and 1000 mg/kg) for 4 weeks | ↓ Lipid deposition, inflammatory infiltrate ↑ Vascularisation of the hepatic tissue ↓ serum ALT and AST activities, nitrite/nitrate, TBARS, and GSH levels ↑ GSH, SOD, CAT, GPx, GR, G6PDH levels in the liver ↓ TBARS level in the liver ↑ mRNA levels of MnSOD, Cu/ZnSOD, GPx1, CAT, and G6DPH in liver ↓ Serum TC, TG, FFA, Phospholipids ↓ Liver TC, TG, FFA, Phospholipids ↓ Inflammatory cytokines TNF-α, IL-10, IL-1β, IL-6 levels in serum ↓ Mitochondrial ROS generation at complex I, complex III, and reverse flow of electrons | [90] | |
| High-fat-diet-induced mice | Mung bean protein isolate (replacement of casein) in diet supplementation for 4 weeks | ↓ Hepatic TG concentration ↑ Plasma TG concentrations ↓ The expression levels of hepatic lipogenic gene: Sterol regulatory element binding transcription factor 1, fatty acid synthase, stearoyl-coenzyme A desaturase 1, glucose-6-phosphate dehydrogenase 2, glucose-6-phosphate dehydrogenase X-linked | [91] | |
| Effect-ethanol induced hepatotoxicity in mice | Vitexin and isovitexin (15 and 13 mg/kg) for 14 days | ↓ Serum ALT, AST ↑ SOD activity ↓ MDA activity ↓ Liver injury | [92] | |
| Effect-ethanol induced hepatotoxicity in mice | Aqueous extracts of germinated and fermented mung beans (200 and 1000 mg/kg) for 7 and 14 days | ↓ Serum ALT, AST, TG, and cholesterol ↑ SOD, FRAP levels in liver ↓ MDA, NO levels in liver ↓ Hepatocyte damage | [52] | |
| Acetaminophen-induced acute hepatotoxicity model in rats | Aqueous extracts of the mung bean (100, 500, and 1000 mg/kg) for a single oral administration | ↓ Serum glutamate-oxalate-transaminase, glutamate-pyruvate-transaminase ↓ Liver damaged by acetaminophen | [93] | |
| Antihypertensive | Spontaneously hypertensive rats | Mung bean protein isolate hydrolysates (600 mg/kg) for a single oral administration | ↓ SBP, heart rate | [94] |
| Spontaneously hypertensive rats | Aqueous extracts and hydrolysates of mung bean sprouts (600 mg peptide/kg) for 8 weeks | ↓ SBD, plasma ACE activity | [95] | |
| Anticancer properties | Breast cancer cells 4T1 injected mice | Aqueous extract of fermented mung bean (200 and 1000 mg/kg) for 30 days | ↓ Tumor formation ↑ Serum anticancer cytokine levels, spleen T cell populations, splenocyte cytotoxicity ↑ IL-2 and IFN-γ ↓ SOD and NO levels in the liver ↓ The lipid peroxidation ↓ Mitotic division in the tumors | [96] |
| Immunomodulatory activity | Cyclophosphamide induced-immunosuppressed model | Verbascose from mung bean (30, 90 and 270 mg/kg) for 8 days | ↑ Spleen and thymus indices ↑ Swelling rate of earlap in the delayed type of hypersensitivity reaction ↑ Serum hemolysin ↑ Lysozyme in Serum and Spleen | [73] |
| Arachidonic acid-induced ear edema in mice | Aqueous extract of untreated, germinated, and fermented mung bean (200 and 1000 mg/kg) for single oral administration | Exhibited the edema inhibition effect | [22] | |
| An animal model of sepsis induced by cecal ligation and puncture | Aqueous extract of mung bean seed coat (0.2 mL/mouse, containing 1.0 mg lyophilized extract) for 2 weeks | ↑ Survival rates | [76] | |
| Complete Freund’s adjuvant-induced arthritis in rats | Ethanolic extract of mung bean (250 and 500 μg/mL) for 21 days | ↑ Body weight, percentage inhibition of paw edema, pain threshold ↓ Serum TNF-α and IL-1β, IL-6 and IL-10 levels ↓ Liver lysosomal enzyme levels (N-acetyl-β-d-glusoaminidase, cathepsin-D, glucuronidase) ↓ MDA levels, myeloperoxidase activity ↑ Glutathione level | [97] | |
| Diabetic KK-Ay mice | Ethanolic extract of mung bean (1 g/kg) for 4 weeks | ↓ Epididymal and perirenal fat weights ↑ Plasma TG and TC levels ↓ Plasma IL-6 levels, ↓ Intramuscular TNF-α and MCP-1 levels ↓ Intramuscular TG and TC levels ↓ The gene expression levels of p-AMPK, ACC, and PGC1α, p-ERK1/2, PPARγ, C/EBPα, and p-p38 in intramuscular | [67] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung Bean (Vigna radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits. Nutrients 2019, 11, 1238. https://doi.org/10.3390/nu11061238
Hou D, Yousaf L, Xue Y, Hu J, Wu J, Hu X, Feng N, Shen Q. Mung Bean (Vigna radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits. Nutrients. 2019; 11(6):1238. https://doi.org/10.3390/nu11061238
Chicago/Turabian StyleHou, Dianzhi, Laraib Yousaf, Yong Xue, Jinrong Hu, Jihong Wu, Xiaosong Hu, Naihong Feng, and Qun Shen. 2019. "Mung Bean (Vigna radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits" Nutrients 11, no. 6: 1238. https://doi.org/10.3390/nu11061238
APA StyleHou, D., Yousaf, L., Xue, Y., Hu, J., Wu, J., Hu, X., Feng, N., & Shen, Q. (2019). Mung Bean (Vigna radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits. Nutrients, 11(6), 1238. https://doi.org/10.3390/nu11061238