Does Beef Protein Supplementation Improve Body Composition and Exercise Performance? A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Search
2.2. Study Selection and Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
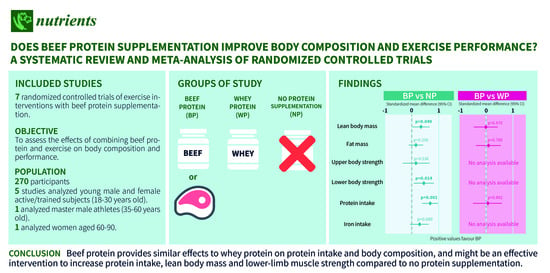
3.1. Included Studies
3.2. Quality Assessment and Publication Bias
3.3. Participants and Intervention Characteristics
3.4. Body Composition
3.5. Exercise Performance
3.6. Nutritional Intake
3.7. Hematological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.M.; Loon, L.J.C. Van Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; McLellan, T.M.; Lieberman, H.R. The Effects of Protein Supplements on Muscle Mass, Strength, and Aerobic and Anaerobic Power in Healthy Adults: A Systematic Review. Sports Med. 2014, 45, 111–131. [Google Scholar] [CrossRef]
- Stokes, T.; Hector, A.J.; Morton, R.W.; McGlory, C.; Phillips, S.M. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 2018, 10, 180. [Google Scholar] [CrossRef]
- Elliot, T.A.; Cree, M.G.; Sanford, A.P.; Wolfe, R.R.; Tipton, K.D. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med. Sci. Sports Exerc. 2006, 38, 667–674. [Google Scholar] [CrossRef]
- Moore, D.; Robinson, M.; Fry, J.; Tang, J.; Glover, E.; Wilkinson, S.; Prior, T.; Tarnopolsky, M.; Philips, S. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 1. [Google Scholar] [CrossRef]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; De Souza, E.O.; Wilson, S.M.; Kalman, D.S.; Dudeck, J.E.; Jäger, R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr. J. 2013, 12, 1–7. [Google Scholar] [CrossRef]
- Duff, W.; Chilibeck, P.; Rooke, J.; Kaviani, M.; Krentz, J.; Haines, D. The Effect of Bovine Colostrum Supplementation in Older Adults during Resistance Training. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 276–285. [Google Scholar] [CrossRef]
- Cribb, P.J.; Williams, A.D.; Hayes, A.; Carey, M.F. The Effect of Whey Isolate and Resistance Training on Strength, Body Composition and Plasma Glutamine. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 494–509. [Google Scholar] [CrossRef]
- Volek, J.S.; Volk, B.M.; Gómez, A.L.; Kunces, L.J.; Kupchak, B.R.; Freidenreich, D.J.; Aristizabal, J.C.; Saenz, C.; Dunn-Lewis, C.; Ballard, K.D.; et al. Whey Protein Supplementation During Resistance Training Augments Lean Body Mass. J. Am. Coll. Nutr. 2013, 32, 122–135. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Rémond, D.; van Loon, L.J.C. The muscle protein synthetic response to food ingestion. Meat Sci. 2015, 109, 96–100. [Google Scholar] [CrossRef]
- Symons, T.; Schutzler, S.E.; Cocke, T.L.; Chinkes, D.L.; Wolfe, R.R.; Paddon-Jones, D. Aging does not impair the anabolic response to a protein-rich meal. Am. J. Clin. Nutr. 2007, 86, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Symons, T.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A Moderate Serving of High-Quality Protein Maximally Stimulates Skeletal Muscle Protein Synthesis in Young and Elderly Subjects. J. Am. Diet. Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.J.; Burd, N.A.; Breen, L.; Rerecich, T.; Yang, Y.; Hector, A.J.; Baker, S.K.; Phillips, S.M. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl. Physiol. Nutr. Metab. 2013, 38, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Symons, T.; Sheffield-Moore, M.; Mamerow, M.M.; Wolfe, R.R.; Paddon-Jones, D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J. Nutr. Health Aging 2011, 15, 376–381. [Google Scholar] [CrossRef]
- Daly, R.M.; O’Connell, S.L.; Mundell, N.L.; Grimes, C.A.; Dunstan, D.W.; Nowson, C.A. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: A cluster randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 899–910. [Google Scholar] [CrossRef]
- Sharp, M.H.; Lowery, R.P.; Shields, K.A.; Lane, J.R.; Gray, J.L.; Partl, J.M.; Hayes, D.W.; Wilson, G.J.; Hollmer, C.A.; Minivich, J.R.; et al. The Effects of Beef, Chicken, or Whey Protein Post-Workout on Body Composition and Muscle Performance. J. Strength Cond. Res. 2018, 32, 2233–2242. [Google Scholar] [CrossRef]
- Naclerio, F.; Larumbe-Zabala, E.; Ashrafi, N.; Seijo, M.; Nielsen, B.; Allgrove, J.; Earnest, C.P. Effects of protein–carbohydrate supplementation on immunity and resistance training outcomes: A double-blind, randomized, controlled clinical trial. Eur. J. Appl. Physiol. 2017, 117, 267–277. [Google Scholar] [CrossRef]
- Burke, D.E.; Johnson, J.V.; Vukovich, M.D.; Kattelmann, K.K. Effects of Lean Beef Supplementation on Iron Status, Body Composition and Performance of Collegiate Distance Runners. Food Nutr. Sci. 2012, 03, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Naclerio, F.; Seijo-Bujia, M.; Larumbe-Zabala, E.; Earnes, C. Carbohydrates Alone or Mixing With Beef or Whey Protein Promote Similar Training Outcomes in Resistance Training Males: A Double Blind, Randomized Controlled Clinical Trial. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, F.; Seijo, M.; Larumbe-Zabala, E.; Ashrafi, N.; Christides, T.; Karsten, B.; Nielsen, B.V. Effects of Supplementation with Beef or Whey Protein Versus Carbohydrate in Master Triathletes. J. Am. Coll. Nutr. 2017, 36, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R. Meta-Analytic Procedures for Social Research; Sage: Newbury Park, CA, USA, 1991. [Google Scholar]
- Negro, M.; Vandoni, M.; Ottobrini, S.; Codrons, E.; Correale, L.; Buonocore, D.; Marzatico, F. Protein supplementation with low fat meat after resistance training: Effects on body composition and strength. Nutrients 2014, 6, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.; Burd, N.A.; van Loon, L.J.C. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef]
- Burd, N.A.; Gorissen, S.H.; Van Vliet, S.; Snijders, T.; Van Loon, L.J.C. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 828–836. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein requirements and optimal intakes in aging: Arewe ready to recommend more than the recommended daily allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; Betz, M.W.; van Loon, L.J.C. The Muscle Protein Synthetic Response to Meal Ingestion Following Resistance-Type Exercise. Sports Med. 2019, 49, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennings, B.; Groen, B.B.L.; Van Dijk, J.W.; De Lange, A.; Kiskini, A.; Kuklinski, M.; Senden, J.M.G.; Van Loon, L.J.C. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am. J. Clin. Nutr. 2013, 98, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Camera, D.M.; Areta, J.L.; Hawley, J. A Beyond muscle hypertrophy: Why dietary protein is important for endurance athletes. Appl. Physiol. Nutr. Metab. 2014, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the “anabolic resistance” of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- MacDiarmid, J.I. Is a healthy diet an environmentally sustainable diet? Proc. Nutr. Soc. 2013, 72, 13–20. [Google Scholar] [CrossRef]
- Scarborough, P.; Appleby, P.N.; Mizdrak, A.; Briggs, A.D.M.; Travis, R.C.; Bradbury, K.E.; Key, T.J. Dietary greenhouse gas emissions of meat-eaters, fish-eaters, vegetarians and vegans in the UK. Clim. Chang. 2014, 125, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, S.H.M.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef]





| Study | Participants and Group Assignment | Duration | Exercise Training Protocol (Common to all Study Groups) | Baseline Protein Intake | BP Group | WP Group | NP Group | Additional Group | Measurements | Main Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Burke et al. [20] | 28 runners (18–24 years, 14 female) stratified by iron status, use of iron supplements, and gender, and randomized into a BP (n = 14) or NP group (n = 14) | 8 weeks | Maintained their typical exercise regime | ~1.7 g/kg/day | 255 g of lean beef supplement per week + multivitamin daily | – | Multivitamin daily | – | -Dietary intake -Blood analysis -Body composition (plethysmography) -VO2max | -No differences in body composition -↑heme iron intake in females -↑hematocrit in females -No differences in VO2max |
| Daly et al. [17] | 100 females (60–90 years) randomized into a BP (n = 53) or NP group (n = 47) | 4 months | RT 2 times per week | ~1.1–1.3 g/kg/day | 220 g lean red meat (45 g protein) 6 days per week + 1 vitamin D3 capsule (1000-IU) daily | – | 1 serving pasta or rice daily (25–35 g CHO) + 1 vitamin D3 capsule (1000-IU) daily | – | -Dietary intake -Physical activity -Body composition (DXA) -Muscle and fat CSA and muscle density (peripheral quantitative computed tomography) -Muscle function (TUG, FSST, and 30-s STS) -Strength (1RM estimated from 3 RM) -Blood analysis | -↑Protein intake -↑LBM -↑Leg LBM -No differences in BMD -↑Muscle strength -No differences in muscle function. -↑IGF-I -↓IL-6 -No differences in blood lipids or blood pressure. |
| Naclerio et al. [21] | 24 active males (~26–29 years) randomized into a BP, WP or NP group (n=8 each) | 8 weeks | RT 3 times per week | ~1.5 g/kg/day | 20 g of beef supplement (16.4 g protein) + 250 mL of orange juice per day | 20 g of WP + 250 mL of orange juice per day | 20 g of CHO + 250 mL of orange juice per day | – | -Body composition (plethysmography) -Limb circumference -Strength (1 RM) -Muscle thickness (ultrasound) | -↑biceps brachialis thickness -No differences in limb circumference or body composition. -No differences in strength. |
| Naclerio et al. [22] | 24 male master triathletes (35–60 years) randomized into a BP, WP or NP group (n = 8 each) | 10 weeks | ET 4–6 times per week | ~1.3–1.5 g/kg/day | 20 g of beef supplement (16.4 g protein) per day | 20 g of WP per day | 20 g of CHO per day | – | -Body composition (plethysmography) -VO2max -Muscle thickness (ultrasound) -Blood analysis | -No differences in body composition. -↓BM -↑heme iron intake. -↑ferritin concentrations -↑muscle thickness -No differences in VO2max |
| Naclerio et al. [19] | 27 active males and females (~24–28 years) randomized into a BP, WP or NP group (n = 8 each) | 8 weeks | RT 3 times per week | ~1.1–1.5 g/kg/day | 20 g of beef supplement (16.4 g protein) + 250 mL of orange juice per day | 20 g of WP + 250 mL of orange juice per day | 20 g of CHO + 250 mL of orange juice per day | – | -Body composition (plethysmography) -Strength (total weight lifted) -Muscle thickness (ultrasound) -Blood analysis -Saliva analysis | -No differences in body composition nor muscle thickness. -↓HNP1-3 and saliva flow rate. -No differences in strength. |
| Negro et al. [26] | 26 male and female healthy subjects (~24 years) randomized into a BP (n = 12) or NP group (n = 14) | 8 weeks | RT 3 times per week | ~1.0 g/kg/day | 135 g (20 g protein) of tinned beef per day | – | No supplement provided | – | -Strength (1 RM) -Body composition (bioimpedance) | -↑FFM and ↓FM -No differences in LBM -No differences in strength. |
| Sharp et al. [18] | 41 male and female trained subjects (18–30 years) randomized into a WP (n = 10, 5 male), BP (n = 10, 5 male), chicken protein (n = 11, 5 male) or NP group (n = 10, 4 male) | 8 weeks | RT 3 times per week and HIIT 2 times per week. | ~2.0–2.2 g/kg/day | 46 g of isolated BP per day | 46 g of WP per day | 46 g of CHO per day | 46 g of chicken protein per day | -Dietary intake -Body composition (DXA). -Anaerobic peak power (10-second sprint) -Strength (1RM) -Gastrointestinal symptoms | -↑LBM and ↓FM compared to CHO. -No differences in body composition compared to other protein sources. -No differences in strength. -↓improvement in peak power compared to WP. -No differences in gastrointestinal symptoms. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela, P.L.; Mata, F.; Morales, J.S.; Castillo-García, A.; Lucia, A. Does Beef Protein Supplementation Improve Body Composition and Exercise Performance? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 1429. https://doi.org/10.3390/nu11061429
Valenzuela PL, Mata F, Morales JS, Castillo-García A, Lucia A. Does Beef Protein Supplementation Improve Body Composition and Exercise Performance? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2019; 11(6):1429. https://doi.org/10.3390/nu11061429
Chicago/Turabian StyleValenzuela, Pedro L., Fernando Mata, Javier S. Morales, Adrián Castillo-García, and Alejandro Lucia. 2019. "Does Beef Protein Supplementation Improve Body Composition and Exercise Performance? A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 11, no. 6: 1429. https://doi.org/10.3390/nu11061429
APA StyleValenzuela, P. L., Mata, F., Morales, J. S., Castillo-García, A., & Lucia, A. (2019). Does Beef Protein Supplementation Improve Body Composition and Exercise Performance? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 11(6), 1429. https://doi.org/10.3390/nu11061429