Selenized Plant Oil Is an Efficient Source of Selenium for Selenoprotein Biosynthesis in Human Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
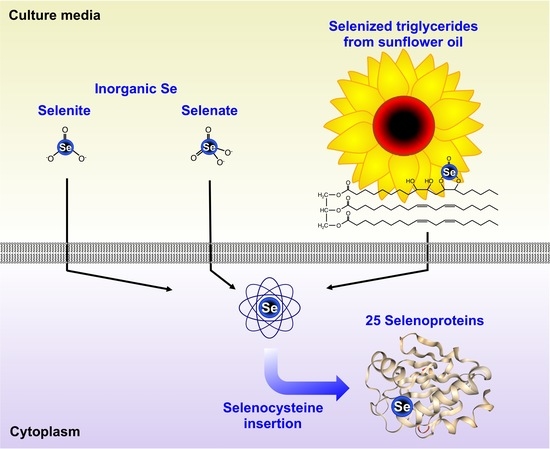
2.2. Cell Culture and Incubation with Different Forms of Selenium
2.3. Measure of Se Levels by ICP-MS
2.4. Evaluation of Selenocysteine Insertion Efficiency
2.5. Protein Gels and Western Immunoblotting
3. Results
3.1. Comparison of the Selenium Uptake by HEK293 from Selol, Selenite and Selenate
3.2. Selol is Able to Stimulate UGA Recoding as Selenocysteine in HEK293 Cells
3.3. Selol Upregulates Selenoprotein Expression Also in LNCaP but not in PNT1A
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigo, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Bulteau, A.-L.; Chavatte, L. Update on selenoprotein biosynthesis. Antioxid. Redox Signal. 2015, 23, 775–794. [Google Scholar] [CrossRef] [PubMed]
- Vindry, C.; Ohlmann, T.; Chavatte, L. Translation regulation of mammalian selenoproteins. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2480–2492. [Google Scholar] [CrossRef] [PubMed]
- Hoefig, C.S.; Renko, K.; Kohrle, J.; Birringer, M.; Schomburg, L. Comparison of different selenocompounds with respect to nutritional value vs. toxicity using liver cells in culture. J. Nutr. Biochem. 2011. [Google Scholar] [CrossRef] [PubMed]
- Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium metabolism, regulation, and sex differences in mammals. In Selenium, Molecular and Integrative Toxicology; Michalke, B., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 89–107. [Google Scholar]
- Suzuki, K.T.; Somekawa, L.; Suzuki, N. Distribution and reuse of 76Se-selenosugar in selenium-deficient rats. Toxicol. Appl. Pharmacol. 2006, 216, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Sonet, J.; Bulteau, A.-L.; Chavatte, L. Selenium and Selenoproteins in Human Health and Diseases. In Metallomics: Analytical Techniques and Speciation Methods; Michalke, B., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 364–381, Chapter 13. [Google Scholar] [CrossRef]
- Touat-Hamici, Z.; Legrain, Y.; Sonet, J.; Bulteau, A.-L.; Chavatte, L. Alteration of selenoprotein expression during stress and in aging. In Selenium: Its Molecular Biology and Role in Human Health, 4th ed.; Hatfield, D.L., Tsuji, P.A., Gladyshev, V.N., Eds.; Springer Science+Business Media, LLC: New York, NY, USA, 2016; pp. 539–551. [Google Scholar]
- Touat-Hamici, Z.; Bulteau, A.L.; Bianga, J.; Jean-Jacques, H.; Szpunar, J.; Lobinski, R.; Chavatte, L. Selenium-regulated hierarchy of human selenoproteome in cancerous and immortalized cells lines. Biochim. Biophys. Acta Gen. Subj. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rebsch, C.M.; Penna, F.J., 3rd.; Copeland, P.R. Selenoprotein expression is regulated at multiple levels in prostate cells. Cell Res. 2006, 16, 940–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allmang, C.; Wurth, L.; Krol, A. The selenium to selenoprotein pathway in eukaryotes: More molecular partners than anticipated. Biochim. Biophys. Acta 2009, 1790, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.M.; Copeland, P.R. Mechanism and regulation of selenoprotein synthesis. Annu. Rev. Nutr. 2003, 23, 17–40. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Gladyshev, V.N. How selenium has altered our understanding of the genetic code. Mol. Cell Biol. 2002, 22, 3565–3576. [Google Scholar] [CrossRef]
- Berry, M.J.; Tujebajeva, R.M.; Copeland, P.R.; Xu, X.M.; Carlson, B.A.; Martin, G.W., 3rd.; Low, S.C.; Mansell, J.B.; Grundner-Culemann, E.; Harney, J.W.; et al. Selenocysteine incorporation directed from the 3‘UTR: Characterization of eukaryotic EFsec and mechanistic implications. Biofactors 2001, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Touat-Hamici, Z.; Legrain, Y.; Bulteau, A.-L.; Chavatte, L. Selective up-regulation of human selenoproteins in response to oxidative stress. J. Biol. Chem. 2014, 289, 14750–14761. [Google Scholar] [CrossRef] [PubMed]
- Latreche, L.; Duhieu, S.; Touat-Hamici, Z.; Jean-Jean, O.; Chavatte, L. The differential expression of glutathione peroxidase 1 and 4 depends on the nature of the SECIS element. RNA Biol. 2012, 9, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominiak, A.; Wilkaniec, A.; Jesko, H.; Czapski, G.A.; Lenkiewicz, A.M.; Kurek, E.; Wroczynski, P.; Adamczyk, A. Selol, an organic selenium donor, prevents lipopolysaccharide-induced oxidative stress and inflammatory reaction in the rat brain. Neurochem. Int. 2017, 108, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Sliwka, L.; Wiktorska, K.; Suchocki, P.; Milczarek, M.; Mielczarek, S.; Lubelska, K.; Cierpial, T.; Lyzwa, P.; Kielbasinski, P.; Jaromin, A.; et al. The Comparison of MTT and CVS Assays for the Assessment of Anticancer Agent Interactions. PLoS ONE 2016, 11, e0155772. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Flis-Borsuk, A.; Suchocki, P.; Szpunar, J.; Lobinski, R. Speciation of Selenium in Selenium-Enriched Sunflower Oil by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry/Electrospray-Orbitrap Tandem Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 4975–4981. [Google Scholar] [CrossRef] [PubMed]
- Flis, A.; Suchocki, P.; Krolikowska, M.A.; Suchocka, Z.; Remiszewska, M.; Sliwka, L.; Ksiazek, I.; Sitarz, K.; Sochacka, M.; Hoser, G.; et al. Selenitetriglycerides-Redox-active agents. Pharmacol. Rep. 2015, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sochacka, M.; Giebultowicz, J.; Remiszewska, M.; Suchocki, P.; Wroczynski, P. Effects of Selol 5% supplementation on the activity or concentration of antioxidants and malondialdehyde level in the blood of healthy mice. Pharmacol. Rep. 2014, 66, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Chavatte, L.; Brown, B.A.; Driscoll, D.M. Ribosomal protein L30 is a component of the UGA-selenocysteine recoding machinery in eukaryotes. Nat. Struct. Mol. Biol. 2005, 12, 408–416. [Google Scholar] [CrossRef]
- Ksiazek, I.; Sitarz, K.; Anuszewska, E.; Dudkiewicz-Wilczynska, J.; Rolson, M.; Koronkiewicz, M.; Suchocki, P. Toxicity studies of selol—An organic selenium (iv) compound- in vitro research. Int. J. Pharm. Pharm. Sci. 2014, 6, 264–269. [Google Scholar]
- Vacchina, V.; Dumont, J. Total Selenium Quantification in Biological Samples by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Methods Mol. Biol. 2018, 1661, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Latreche, L.; Jean-Jean, O.; Driscoll, D.M.; Chavatte, L. Novel structural determinants in human SECIS elements modulate the translational recoding of UGA as selenocysteine. Nucleic Acids Res. 2009, 37, 5868–5880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrain, Y.; Touat-Hamici, Z.; Chavatte, L. Interplay between selenium levels, selenoprotein expression, and replicative senescence in WI-38 human fibroblasts. J. Biol. Chem. 2014, 289, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Szpunar, J.; Lobinski, R. Biological Selenium Species and Selenium Speciation in Biological Samples. In Selenium: Its Molecular Biology and Role in Human Health, 4th ed.; Hatfield, D.L., Tsuji, P.A., Gladyshev, V.N., Eds.; Springer Science+Business Media, LLC: New York, NY, USA, 2016; pp. 413–424. [Google Scholar] [CrossRef]
- Ramoutar, R.R.; Brumaghim, J.L. Antioxidant and anticancer properties and mechanisms of inorganic selenium, oxo-sulfur, and oxo-selenium compounds. Cell Biochem. Biophys. 2010, 58, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, I.; Sitarz, K.; Roslon, M.; Anuszewska, E.; Suchocki, P.; Wilczynska, J.D. The influence of Selol on the expression of oxidative stress genes in normal and malignant prostate cells. Cancer Genom. Proteom. 2013, 10, 225–232. [Google Scholar]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonet, J.; Mosca, M.; Bierla, K.; Modzelewska, K.; Flis-Borsuk, A.; Suchocki, P.; Ksiazek, I.; Anuszewska, E.; Bulteau, A.-L.; Szpunar, J.; et al. Selenized Plant Oil Is an Efficient Source of Selenium for Selenoprotein Biosynthesis in Human Cell Lines. Nutrients 2019, 11, 1524. https://doi.org/10.3390/nu11071524
Sonet J, Mosca M, Bierla K, Modzelewska K, Flis-Borsuk A, Suchocki P, Ksiazek I, Anuszewska E, Bulteau A-L, Szpunar J, et al. Selenized Plant Oil Is an Efficient Source of Selenium for Selenoprotein Biosynthesis in Human Cell Lines. Nutrients. 2019; 11(7):1524. https://doi.org/10.3390/nu11071524
Chicago/Turabian StyleSonet, Jordan, Maurine Mosca, Katarzyna Bierla, Karolina Modzelewska, Anna Flis-Borsuk, Piotr Suchocki, Iza Ksiazek, Elzbieta Anuszewska, Anne-Laure Bulteau, Joanna Szpunar, and et al. 2019. "Selenized Plant Oil Is an Efficient Source of Selenium for Selenoprotein Biosynthesis in Human Cell Lines" Nutrients 11, no. 7: 1524. https://doi.org/10.3390/nu11071524
APA StyleSonet, J., Mosca, M., Bierla, K., Modzelewska, K., Flis-Borsuk, A., Suchocki, P., Ksiazek, I., Anuszewska, E., Bulteau, A.-L., Szpunar, J., Lobinski, R., & Chavatte, L. (2019). Selenized Plant Oil Is an Efficient Source of Selenium for Selenoprotein Biosynthesis in Human Cell Lines. Nutrients, 11(7), 1524. https://doi.org/10.3390/nu11071524