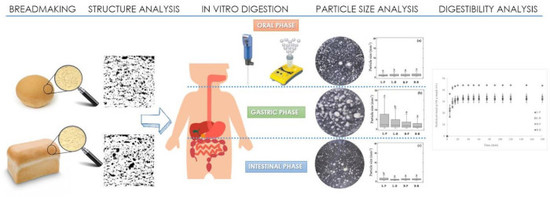
Effect of Bread Structure and In Vitro Oral Processing Methods in Bolus Disintegration and Glycemic Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bread Preparation
2.3. Bread Characterization
2.4. In Vitro Oro-Gastro-Intestinal Digestion
2.5. Reducing Sugars Released and In Vitro Starch Digestibility
2.6. Particle Size Distribution of the Bolus during In Vitro Digestion
2.7. Statistical Analyses
3. Results
3.1. Variation of Bread Structure as a Consequence of Changes in the Breadmaking Process
3.2. Bolus Particle Size throughout In Vitro Digestion
3.3. In Vitro Digestion and Expected Glycemic Index
3.4. Multivariable Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266–273. [Google Scholar] [CrossRef]
- Granfeldt, Y.; Björck, I.; Drews, A.; Tovar, J. An in vitro procedure based on chewing to predict metabolic response to starch in cereal and legume products. Am. J. Clin. Nutr. 1992, 46, 649–660. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Rioux, L.-E. Food matrix impact on macronutrients nutritional properties. Food Hydrocoll. 2011, 25, 1915–1924. [Google Scholar] [CrossRef]
- Gao, J.; Lin, S.; Jin, X.; Wang, Y.; Ying, J.; Dong, Z.; Zhou, W. In vitro digestion of bread: How is it influenced by the bolus characteristics? J. Texture Stud. 2019, 50, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Rémésy, C. Parameters controlling the glycaemic response to breads. Nutr. Res. Rev. 2007, 19, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wong, J.X.; Lim, J.C.-S.; Henry, J.; Zhou, W. Influence of bread structure on human oral processing. J. Food Eng. 2015, 167, 147–155. [Google Scholar] [CrossRef]
- Eelderink, C.; Noort, M.W.J.; Sozer, N.; Koehorst, M.; Holst, J.J.; Deacon, C.F.; Rehfeld, J.F.; Poutanen, K.; Vonk, R.J.; Oudhuis, L.; et al. The structure of wheat bread influences the postprandial metabolic response in healthy men. Food Funct. 2015, 6, 3236–3248. [Google Scholar] [CrossRef]
- Borczak, B.; Sikora, M.; Sikora, E.; Dobosz, A.; Kapusta-Duch, J. Glycaemic index of wheat bread. Starch-Stärke 2018, 70, 1700022. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Fornés-Ferrer, V.; Heredia, A.; Andrés, A. In Vitro Digestion of Lipids in Real Foods: Influence of Lipid Organization Within the Food Matrix and Interactions with Nonlipid Components. J. Food Sci. 2018, 83, 2629–2637. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.E.; Rosell, C.M. Relationship between instrumental parameters and sensory characteristics in gluten-free breads. Eur. Food Res. Technol. 2012, 235, 107–117. [Google Scholar] [CrossRef] [Green Version]
- International Association for Cereal Science and Technology. ICC-Standard No 110/1. Approved 1960, Revised 1976. Available online: https://www.icc.or.at/publications/icc-standards/standards-overview/110-1-determination-of-the-moisture-content-of-cereals-and-cereal-products-practical-method (accessed on 21 May 2019).
- Morreale, F.; Garzón, R.; Rosell, C.M. Understanding the role of hydrocolloids viscosity and hydration in developing gluten-free bread. A study with hydroxypropylmethylcellulose. Food Hydrocoll. 2018, 77, 629–635. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Le Bleis, F.; Chaunier, L.; Della Valle, G.; Panouillé, M.; Réguerre, A.L. Physical assessment of bread destructuration during chewing. Food Res. Int. 2013, 50, 308–317. [Google Scholar] [CrossRef]
- Cauvain, S. Breadmaking Processes. In Technology of Breadmaking; Springer International Publishing: Cham, Switzerland, 2015; pp. 23–55. [Google Scholar]
- Chen, J. Food oral processing: Mechanisms and implications of food oral destruction. Trends Food Sci. Technol. 2015, 45, 222–228. [Google Scholar] [CrossRef]
- Mosca, A.C.; Chen, J. Food oral management: Physiology and objective assessment. Curr. Opin. Food Sci. 2016, 9, 11–20. [Google Scholar] [CrossRef]
- Jalabert-Malbos, M.-L.; Mishellany-Dutour, A.; Woda, A.; Peyron, M.-A. Particle size distribution in the food bolus after mastication of natural foods. Food Qual. Prefer. 2007, 18, 803–812. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Ferrua, M.J. Aspects of food structures in the digestive tract. Curr. Opin. Food Sci. 2015, 3, 85–93. [Google Scholar] [CrossRef]
- Bornhorst, G.; Singh, R. Kinetics of in Vitro Bread Bolus Digestion with Varying Oral and Gastric Digestion Parameters. Food Biophys. 2013, 8, 50–59. [Google Scholar] [CrossRef]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P.; Wattanakorn, N.; Takeuchi, H. Study on the mucoadhesion mechanism of pectin by atomic force microscopy and mucin-particle method. Carbohydr. Polym. 2010, 79, 54–59. [Google Scholar] [CrossRef]
- Goñi, I.; Valdivieso, L.; Gudiel-Urbano, M. Capacity of edible seaweeds to modify in vitro starch digestibility of wheat bread. Food/Nahrung 2002, 46, 18–20. [Google Scholar] [CrossRef]
- Björck, I.; Nyman, M.; Pedersen, B.; Siljeström, M.; Asp, N.G.; Eggum, B.O. On the digestibility of starch in wheat bread—Studies in vitro and in vivo. J. Cereal Sci. 1986, 4, 1–11. [Google Scholar] [CrossRef]
- Woolnough, J.W.; Bird, A.R.; Monro, J.A.; Brennan, C.S. The Effect of a Brief Salivary α-Amylase Exposure During Chewing on Subsequent in Vitro Starch Digestion Curve Profiles. Int. J. Mol. Sci. 2010, 11, 2780–2790. [Google Scholar] [CrossRef]





| Loaf Bread (L) | Bread Roll (B) | |
|---|---|---|
| Moisture (%) | 28.18 ± 2.84 a | 36.73 ± 3.28 b |
| Slice 2D Area (cm2) | 30.86 ± 0.68 a | 42.06 ± 1.77 b |
| Cell area (mm2) | 0.26 ± 0.01 b | 0.23 ± 0.01 a |
| Porosity (%) | 24.38 ± 1.67 a | 33.88 ± 2.06 b |
| Hardness (N) | 1.18 ± 0.10 a | 2.69 ± 0.14 b |
| Cohesiveness | 0.95 ± 0.02 b | 0.86 ± 0.03 a |
| Chewiness | 1.02 ± 0.02 a | 2.74 ± 0.79 b |
| Resilience | 0.54 ± 0.02 b | 0.43 ± 0.04 a |
| Springiness | 0.85 ± 0.08 a | 0.94 ± 0.04 b |
| Shaping Method | Oral Processing Method | k | C∞ A | AUC | HI | eGI B |
|---|---|---|---|---|---|---|
| L | P | 0.24 ± 0.05 | 31.94 ± 2.88 a | 5600 ± 532 a | 70.14 ± 6.67 a | 64.86 ± 2.31 a |
| B | 0.27 ± 0.06 | 32.66 ± 0.12 a | 5736 ± 48 a | 71.85 ± 0.6 a | 65.44 ± 0.10 a | |
| B | P | 0.13 ± 0.01 | 34.31 ± 2.18 a | 5906 ± 355 a | 73.98 ± 4.45 a | 66.76 ± 1.75 a |
| B | 0.27 ± 0.14 | 44.15 ± 0.42 b | 7712 ± 42 b | 96.6 ± 0.52 b | 74.66 ± 0.34 b | |
| P-value | Shaping method | 0.3880 | 0.0316 | 0.0436 | 0.0436 | 0.0317 |
| Oral processing method | 0.2218 | 0.0738 | 0.0771 | 0.0771 | 0.0712 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleixandre, A.; Benavent-Gil, Y.; Rosell, C.M. Effect of Bread Structure and In Vitro Oral Processing Methods in Bolus Disintegration and Glycemic Index. Nutrients 2019, 11, 2105. https://doi.org/10.3390/nu11092105
Aleixandre A, Benavent-Gil Y, Rosell CM. Effect of Bread Structure and In Vitro Oral Processing Methods in Bolus Disintegration and Glycemic Index. Nutrients. 2019; 11(9):2105. https://doi.org/10.3390/nu11092105
Chicago/Turabian StyleAleixandre, Andrea, Yaiza Benavent-Gil, and Cristina M. Rosell. 2019. "Effect of Bread Structure and In Vitro Oral Processing Methods in Bolus Disintegration and Glycemic Index" Nutrients 11, no. 9: 2105. https://doi.org/10.3390/nu11092105
APA StyleAleixandre, A., Benavent-Gil, Y., & Rosell, C. M. (2019). Effect of Bread Structure and In Vitro Oral Processing Methods in Bolus Disintegration and Glycemic Index. Nutrients, 11(9), 2105. https://doi.org/10.3390/nu11092105