Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Study Selection
3.2. Characteristics of Included Studies
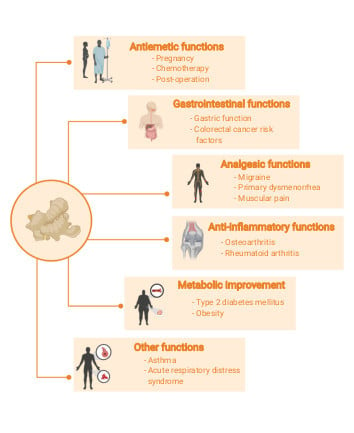
3.3. Clinical Effects of Ginger
3.3.1. Antiemetic Function
3.3.2. Gastrointestinal Function
3.3.3. Analgesic Function
3.3.4. Inflammatory Effect
3.3.5. Metabolic Improvement
3.3.6. Other Clinical Functions
3.4. Adverse Effects
3.5. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, H.; Liu, Y.; Luo, D.; Ma, Y.; Zhang, J.; Li, M.; Yao, L.; Shi, X.; Liu, X.; Yang, K. Ginger for health care: An overview of systematic reviews. Complement. Ther. Med. 2019, 45, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Weimer, K.; Schulte, J.; Maichle, A.; Muth, E.R.; Scisco, J.L.; Horing, B.; Enck, P.; Klosterhalfen, S. Effects of ginger and expectations on symptoms of nausea in a balanced placebo design. PLoS ONE 2012, 7, e49031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensiyeh, J.; Sakineh, M.-A.C. Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: A randomised controlled trial. Midwifery 2009, 25, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, F.; Kashanian, M.; Koohpayehzadeh, J.; Rezaian, F.; Sheikhansari, N.; Eshraghi, N. A comparison between the effects of ginger, pyridoxine (vitamin B6) and placebo for the treatment of the first trimester nausea and vomiting of pregnancy (NVP). J. Matern. Fetal Neonatal Med. 2018, 31, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Kubra, I.R.; Rao, L.J.M. An impression on current developments in the technology, chemistry, and biological activities of ginger (Zingiber officinale Roscoe). Crit. Rev. Food Sci. Nutr. 2012, 52, 651–688. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crop. Prod. 2015, 70, 238–244. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Crichton, M.; Marshall, S.; Marx, W.; McCarthy, A.L.; Isenring, E. Efficacy of ginger (Zingiber officinale) in ameliorating chemotherapy-induced nausea and vomiting and chemotherapy-related outcomes: A systematic review update and meta-analysis. J. Acad. Nutr. Diet. 2019. [Google Scholar] [CrossRef]
- Chen, C.X.; Barrett, B.; Kwekkeboom, K.L. Efficacy of oral ginger (Zingiber officinale) for dysmenorrhea: A systematic review and meta-analysis. Evid.-Based Complement. Altern. Med. 2016, 2016, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Chen, H.; Song, Z.; Wang, X.; Sun, Z. Effects of ginger (Zingiber officinale Roscoe) on type 2 diabetes mellitus and components of the metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Evid.-Based Complement. Altern. Med. 2018, 2018, 5692962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffari-Khosravi, H.; Naderi, Z.; Dehghan, A.; Nadjarzadeh, A.; Fallah Huseini, H. Effect of ginger supplementation on proinflammatory cytokines in older patients with osteoarthritis: Outcomes of a randomized controlled clinical trial. J. Nutr. Gerontol. Geriatr. 2016, 35, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Turgeon, D.K.; Wright, B.D.; Sidahmed, E.; Ruffin, M.T.; Brenner, D.E.; Sen, A.; Zick, S.M. Effect of ginger root on cyclooxygenase-1 and 15-hydroxyprostaglandin dehydrogenase expression in colonic mucosa of humans at normal and increased risk for colorectal cancer. Eur. J. Cancer Prev. 2013, 22, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Marx, W.; McCarthy, A.L.; Ried, K.; McKavanagh, D.; Vitetta, L.; Sali, A.; Lohning, A.; Isenring, E. The effect of a standardized ginger extract on chemotherapy-induced nausea-related quality of life in patients undergoing moderately or highly emetogenic chemotherapy: A double blind, randomized, placebo controlled trial. Nutrients 2017, 9, 867. [Google Scholar] [CrossRef]
- Sanaati, F.; Najafi, S.; Kashaninia, Z.; Sadeghi, M. Effect of ginger and chamomile on nausea and vomiting caused by chemotherapy in Iranian women with breast cancer. Asian Pac. J. Cancer Prev. 2016, 17, 4125–4129. [Google Scholar]
- Thamlikitkul, L.; Srimuninnimit, V.; Akewanlop, C.; Ithimakin, S.; Techawathanawanna, S.; Korphaisarn, K.; Chantharasamee, J.; Danchaivijitr, P.; Soparattanapaisarn, N. Efficacy of ginger for prophylaxis of chemotherapy-induced nausea and vomiting in breast cancer patients receiving adriamycin-cyclophosphamide regimen: A randomized, double-blind, placebo-controlled, crossover study. Support Care Cancer 2017, 25, 459–464. [Google Scholar] [CrossRef]
- Li, X.; Qin, Y.; Liu, W.; Zhou, X.Y.; Li, Y.N.; Wang, L.Y. Efficacy of ginger in ameliorating acute and delayed chemotherapy-induced nausea and vomiting among patients with lung cancer receiving cisplatin-based regimens: A randomized controlled trial. Integr. Cancer 2018, 17, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.; Porouhan, P.; Mohammadianpanah, M.; Omidvari, S.; Mosalaei, A.; Ahmadloo, N.; Nasrollahi, H.; Hamedi, S.H. Efficacy of ginger in control of chemotherapy induced nausea and vomiting in breast cancer patients receiving doxorubicin-based chemotherapy. Asian Pac. J. Cancer Prev. 2016, 17, 3877–3880. [Google Scholar] [PubMed]
- Matsumura, M.D.; Zavorsky, G.S.; Smoliga, J.M. The effects of pre-exercise ginger supplementation on muscle damage and delayed onset muscle soreness. Phytother. Res. 2015, 29, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.B.; Rodrigues, A.; Rodrigues, D.F.; Dos Santos, L.C.; Teixeira, A.L.; Ferreira, A.V.M. Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc) addition in migraine acute treatment. Cephalalgia 2019, 39, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Makhdoomi Arzati, M.; Mohammadzadeh Honarvar, N.; Saedisomeolia, A.; Anvari, S.; Effatpanah, M.; Makhdoomi Arzati, R.; Yekaninejad, M.S.; Hashemi, R.; Djalali, M. The effects of ginger on fasting blood sugar, hemoglobin A1c, and lipid profiles in patients with type 2 diabetes. Int. J. Endocrinol. Metab. 2017, 15, e57927. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimzadeh Attari, V.; Ostadrahimi, A.; Asghari Jafarabadi, M.; Mehralizadeh, S.; Mahluji, S. Changes of serum adipocytokines and body weight following zingiber officinale supplementation in obese women: A RCT. Eur. J. Nutr. 2016, 55, 2129–2136. [Google Scholar] [CrossRef]
- Ebrahimzadeh Attari, V.; Asghari Jafarabadi, M.; Zemestani, M.; Ostadrahimi, A. Effect of zingiber officinale supplementation on obesity management with respect to the uncoupling protein 1 -3826A>G and ß3-adrenergic receptor Trp64Arg polymorphism. Phytother. Res. 2015, 29, 1032–1039. [Google Scholar] [CrossRef]
- Aryaeian, N.; Shahram, F.; Mahmoudi, M.; Tavakoli, H.; Yousefi, B.; Arablou, T.; Jafari Karegar, S. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active rheumatoid arthritis. Gene 2019, 698, 179–185. [Google Scholar] [CrossRef]
- Kashefi, F.; Khajehei, M.; Alavinia, M.; Golmakani, E.; Asili, J. Effect of ginger (Zingiber officinale) on heavy menstrual bleeding: A placebo-controlled, randomized clinical trial. Phytother. Res. 2015, 29, 114–119. [Google Scholar] [CrossRef]
- Paritakul, P.; Ruangrongmorakot, K.; Laosooksathit, W.; Suksamarnwong, M.; Puapornpong, P. The effect of ginger on breast milk volume in the early postpartum period: A randomized, double-blind controlled trial. Breastfeed. Med. 2016, 11, 361–365. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Roscoe, J.A.; Dakhil, S.R.; Kirshner, J.; Flynn, P.J.; Hickok, J.T.; Morrow, G.R. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: A URCC CCOP study of 576 patients. Support Care Cancer 2012, 20, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Zick, S.M.; Ruffin, M.T.; Lee, J.; Normolle, D.P.; Siden, R.; Alrawi, S.; Brenner, D.E. Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer 2009, 17, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahimi, F.; Khodadad, K.; Amini, S.; Naghibi, F.; Salamzadeh, J.; Baniasadi, S. Evaluating the effect of zingiber officinalis on nausea and vomiting in patients receiving cisplatin based regimens. Iran. J. Pharm. Res. 2011, 10, 379–384. [Google Scholar] [PubMed]
- Yekta, Z.P.; Ebrahimi, S.M.; Hosseini, M.; Nasrabadi, A.N.; Sedighi, S.; Surmaghi, M.H.; Madani, H. Ginger as a miracle against chemotherapy-induced vomiting. Iran. J. Nurs. Midwifery Res. 2012, 17, 325–329. [Google Scholar] [PubMed]
- Willetts, K.E.; Ekangaki, A.; Eden, J.A. Effect of a ginger extract on pregnancy-induced nausea: A randomised controlled trial. Aust. N. Z. J. Obstet. Gynaecol. 2003, 43, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Vutyavanich, T.; Kraisarin, T.; Ruangsri, R. Ginger for nausea and vomiting in pregnancy: Randomized, double-masked, placebo-controlled trial. Obstet. Gynecol. 2001, 97, 577–582. [Google Scholar] [CrossRef]
- Fischer-Rasmussen, W.; Kjaer, S.K.; Dahl, C.; Asping, U. Ginger treatment of hyperemesis gravidarum. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 38, 19–24. [Google Scholar] [CrossRef]
- Smith, C.; Crowther, C.; Willson, K.; Hotham, N.; McMillian, V. A randomized controlled trial of ginger to treat nausea and vomiting in pregnancy. Obstet. Gynecol. 2004, 103, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.C.; Dey, R.; Kamliya, G.S.; Bal, R.; Hazra, A.; Tripathi, S. A single-masked, randomized, controlled trial of ginger extract in the treatment of nausea and vomiting of pregnancy. J. Int. Med. Sci. Acad. 2011, 24, 167–169. [Google Scholar]
- Firouzbakht, M.; Nikpour, M.; Jamali, B.; Omidvar, S. Comparison of ginger with vitamin B6 in relieving nausea and vomiting during pregnancy. Ayu 2014, 35, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Arfeen, Z.; Owen, H.; Plummer, J.L.; Ilsley, A.H.; Sorby-Adams, R.A.; Doecke, C.J. A double-blind randomized controlled trial of ginger for the prevention of postoperative nausea and vomiting. Anaesth. Intensive Care 1995, 23, 449–452. [Google Scholar] [CrossRef] [Green Version]
- Eberhart, L.H.; Mayer, R.; Betz, O.; Tsolakidis, S.; Hilpert, W.; Morin, A.M.; Geldner, G.; Wulf, H.; Seeling, W. Ginger does not prevent postoperative nausea and vomiting after laparoscopic surgery. Anesth. Analg. 2003, 96, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Das, A.; Majumdar, S.; Bhattacharyya, T.; Mitra, T.; Kundu, R. The efficacy of ginger added to ondansetron for preventing postoperative nausea and vomiting in ambulatory surgery. Pharmacogn. Res 2014, 6, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Ozgoli, G.; Goli, M.; Moattar, F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J. Altern. Complement. Med. 2009, 15, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashefi, F.; Khajehei, M.; Tabatabaeichehr, M.; Alavinia, M.; Asili, J. Comparison of the effect of ginger and zinc sulfate on primary dysmenorrhea: A placebo-controlled randomized trial. Pain Manag. Nurs. 2014, 15, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, P.; Montazeri, A.; Huseini, H.F.; Kianbakht, S.; Naseri, M. Effect of zingiber officinale R. rhizomes (ginger) on pain relief in primary dysmenorrhea: A placebo randomized trial. BMC Complement. Altern. Med. 2012, 12, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, C.D.; O’Connor, P.J. Acute effects of dietary ginger on muscle pain induced by eccentric exercise. Phytother. Res. 2010, 24, 1620–1626. [Google Scholar] [CrossRef]
- Black, C.D.; Herring, M.P.; Hurley, D.J.; O’Connor, P.J. Ginger (Zingiber officinale) reduces muscle pain caused by eccentric exercise. J. Pain 2010, 11, 894–903. [Google Scholar] [CrossRef]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef]
- Khandouzi, N.; Shidfar, F.; Rajab, A.; Rahideh, T.; Hosseini, P.; Mir Taheri, M. The effects of ginger on fasting blood sugar, hemoglobin a1c, apolipoprotein B, apolipoprotein a-I and malondialdehyde in type 2 diabetic patients. Iran. J. Pharm. Res. 2015, 14, 131–140. [Google Scholar]
- Phillips, S.; Hutchinson, S.; Ruggier, R. Zingiber officinale does not affect gastric emptying rate. A randomised, placebo-controlled, crossover trial. Anaesthesia 1993, 48, 393–395. [Google Scholar] [CrossRef]
- Citronberg, J.; Bostick, R.; Ahearn, T.; Turgeon, D.K.; Ruffin, M.T.; Djuric, Z.; Sen, A.; Brenner, D.E.; Zick, S.M. Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: Results from a pilot, randomized, and controlled trial. Cancer Prev. Res. 2013, 6, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zick, S.M.; Turgeon, D.K.; Vareed, S.K.; Ruffin, M.T.; Litzinger, A.J.; Wright, B.D.; Alrawi, S.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Phase II study of the effects of ginger root extract on eicosanoids in colon mucosa in people at normal risk for colorectal cancer. Cancer Prev. Res. 2011, 4, 1929–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zick, S.M.; Turgeon, D.K.; Ren, J.; Ruffin, M.T.; Wright, B.D.; Sen, A.; Djuric, Z.; Brenner, D.E. Pilot clinical study of the effects of ginger root extract on eicosanoids in colonic mucosa of subjects at increased risk for colorectal cancer. Mol. Carcinog. 2015, 54, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Van Tilburg, M.A.L.; Palsson, O.S.; Ringel, Y.; Whitehead, W.E. Is ginger effective for the treatment of irritable bowel syndrome? A double blind randomized controlled pilot trial. Complement. Ther. Med. 2014, 22, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigler, I.; Grotto, I.; Caspi, D.; Yaron, M. The effects of zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthr. Cartil. 2003, 11, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Pillai, A.K.; Sharma, K.K.; Gupta, Y.K.; Bakhshi, S. Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatr. Blood Cancer 2011, 56, 234–238. [Google Scholar] [CrossRef]
- Konmun, J.; Danwilai, K.; Ngamphaiboon, N.; Sripanidkulchai, B.; Sookprasert, A.; Subongkot, S. A phase II randomized double-blind placebo-controlled study of 6-gingerol as an anti-emetic in solid tumor patients receiving moderately to highly emetogenic chemotherapy. Med. Oncol. 2017, 34, 69. [Google Scholar] [CrossRef]
- Apariman, S.; Ratchanon, S.; Wiriyasirivej, B. Effectiveness of ginger for prevention of nausea and vomiting after gynecological laparoscopy. J. Med. Assoc. Thai. 2006, 89, 2003–2009. [Google Scholar]
- Chaiyakunapruk, N.; Kitikannakorn, N.; Nathisuwan, S.; Leeprakobboon, K.; Leelasettagool, C. The efficacy of ginger for the prevention of postoperative nausea and vomiting: A meta-analysis. Am. J. Obstet. Gynecol. 2006, 194, 95–99. [Google Scholar] [CrossRef]
- Phillips, S.; Ruggier, R.; Hutchinson, S.E. Zingiber officinale (ginger)—An antiemetic for day case surgery. Anaesthesia 1993, 48, 715–717. [Google Scholar] [CrossRef]
- Bone, M.E.; Wilkinson, D.J.; Young, J.R.; McNeil, J.; Charlton, S. Ginger root—A new antiemetic. The effect of ginger root on postoperative nausea and vomiting after major gynaecological surgery. Anaesthesia 1990, 45, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Dabaghzadeh, F.; Khalili, H.; Dashti-Khavidaki, S.; Abbasian, L.; Moeinifard, A. Ginger for prevention of antiretroviral-induced nausea and vomiting: A randomized clinical trial. Expert Opin. Drug Saf. 2014, 13, 859–866. [Google Scholar] [CrossRef]
- Lien, H.C.; Sun, W.M.; Chen, Y.H.; Kim, H.; Hasler, W.; Owyang, C. Effects of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G481–G489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonlachanvit, S.; Chen, Y.H.; Hasler, W.L.; Sun, W.M.; Owyang, C. Ginger reduces hyperglycemia-evoked gastric dysrhythmias in healthy humans: Possible role of endogenous prostaglandins. J. Pharm. Exp. 2003, 307, 1098–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirvani, M.A.; Motahari-Tabari, N.; Alipour, A. Use of ginger versus stretching exercises for the treatment of primary dysmenorrhea: A randomized controlled trial. J. Integr. Med. 2017, 15, 295–301. [Google Scholar] [CrossRef]
- Maghbooli, M.; Golipour, F.; Moghimi Esfandabadi, A.; Yousefi, M. Comparison between the efficacy of ginger and sumatriptan in the ablative treatment of the common migraine. Phytother. Res. 2014, 28, 412–415. [Google Scholar] [CrossRef]
- Kulkarni, R.A.; Deshpande, A.R. Anti-inflammatory and antioxidant effect of ginger in tuberculosis. J. Complement. Integr. Med. 2016, 13, 201–206. [Google Scholar] [CrossRef]
- Bordia, A.; Verma, S.K.; Srivastava, K.C. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot. Essent. Fat. Acids 1997, 56, 379–384. [Google Scholar] [CrossRef]
- Marx, W.; Ried, K.; McCarthy, A.L.; Vitetta, L.; Sali, A.; McKavanagh, D.; Isenring, L. Ginger—Mechanism of action in chemotherapy-induced nausea and vomiting: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Danwilai, K.; Konmun, J.; Sripanidkulchai, B.-O.; Subongkot, S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: A pilot study. Cancer Manag. Res. 2017, 9, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Drozdov, V.N.; Kim, V.A.; Tkachenko, E.V.; Varvanina, G.G. Influence of a specific ginger combination on gastropathy conditions in patients with osteoarthritis of the knee or hip. J. Altern. Complement. Med. 2012, 18, 583–588. [Google Scholar] [CrossRef]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Saneei Totmaj, A.; Emamat, H.; Jarrahi, F.; Zarrati, M. The effect of ginger (Zingiber officinale) on chemotherapy-induced nausea and vomiting in breast cancer patients: A systematic literature review of randomized controlled trials. Phytother. Res. 2019, 33, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.M.; Teleni, L.; McCarthy, A.L.; Vitetta, L.; McKavanagh, D.; Thomson, D.; Isenring, E. Ginger (Zingiber officinale) and chemotherapy-induced nausea and vomiting: A systematic literature review. Nutr. Rev. 2013, 71, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahi, Y.; Saadat, A.; Sahebkar, A.; Hashemian, F.; Taghikhani, M.; Abolhasani, E. Effect of ginger on acute and delayed chemotherapy-induced nausea and vomiting: A pilot, randomized, open-label clinical trial. Integr. Cancer 2012, 11, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Ozdemir, L. Oral intake of ginger for chemotherapy-induced nausea and vomiting among women with breast cancer. Clin. J. Oncol. Nurs. 2015, 19, E92–E97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portnoi, G.; Chng, L.-A.; Karimi-Tabesh, L.; Koren, G.; Tan, M.P.; Einarson, A. Prospective comparative study of the safety and effectiveness of ginger for the treatment of nausea and vomiting in pregnancy. Am. J. Obstet. Gynecol. 2003, 189, 1374–1377. [Google Scholar] [CrossRef]
- Heitmann, K.; Nordeng, H.; Holst, L. Safety of ginger use in pregnancy: Results from a large population-based cohort study. Eur. J. Clin. Pharmacol. 2013, 69, 269–277. [Google Scholar] [CrossRef]
- Stanisiere, J.; Mousset, P.-Y.; Lafay, S. How safe is ginger rhizome for decreasing nausea and vomiting in women during early pregnancy? Foods 2018, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Kalava, A.; Darji, S.J.; Kalstein, A.; Yarmush, J.M.; SchianodiCola, J.; Weinberg, J. Efficacy of ginger on intraoperative and postoperative nausea and vomiting in elective cesarean section patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 184–188. [Google Scholar] [CrossRef]
- Gillart, T.; Bazin, J.E.; Montetagaud, M.; Bevillard, F.; Amara, S.; Schoeffler, P. The effects of volume and speed of injection in peribulbar anaesthesia. Anaesthesia 1998, 53, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 11. [Google Scholar] [CrossRef] [Green Version]
- Paramdeep, G. Efficacy and tolerability of ginger (Zingiber officinale) in patients of osteoarthritis of knee. Indian J. Physiol. Pharm. 2013, 57, 177–183. [Google Scholar]
- Bliddal, H.; Rosetzsky, A.; Schlichting, P.; Weidner, M.S.; Andersen, L.A.; Ibfelt, H.H.; Christensen, K.; Jensen, O.N.; Barslev, J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthr. Cartil. 2000, 8, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Aryaeian, N.; Valizadeh, M.; Sharifi, F.; Hosseini, A.; Djalali, M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Food Sci. Nutr. 2014, 65, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Talaei, B.; Jalali, B.-A.; Najarzadeh, A.; Mozayan, M.R. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2014, 22, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tabibi, H.; Imani, H.; Atabak, S.; Najafi, I.; Hedayati, M.; Rahmani, L. Effects of ginger on serum lipids and lipoproteins in peritoneal dialysis patients: A randomized controlled trial. Perit. Dial. Int. 2016, 36, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Andallu, B.; Radhika, B.; Suryakantham, V. Effect of aswagandha, ginger and mulberry on hyperglycemia and hyperlipidemia. Plant Foods Hum. Nutr. 2003, 58, 1–7. [Google Scholar] [CrossRef]
- Karimi, N.; Dabidi Roshan, V.; Fathi Bayatiyani, Z. Individually and combined water-based exercise with ginger supplement, on systemic inflammation and metabolic syndrome indices, among the obese women with breast neoplasms. Iran. J. Cancer Prev. 2015, 8, e3856. [Google Scholar] [CrossRef] [Green Version]
- Adib Rad, H.; Basirat, Z.; Bakouei, F.; Moghadamnia, A.A.; Khafri, S.; Farhadi Kotenaei, Z.; Nikpour, M.; Kazemi, S. Effect of ginger and novafen on menstrual pain: A cross-over trial. Taiwan J. Obstet. Gynecol. 2018, 57, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Cortinovis, D.; Fatigoni, S.; Cossu Rocca, M.; Fabi, A.; Seminara, P.; Ripamonti, C.; Alfieri, S.; Granata, R.; Bergamini, C.; et al. A randomized, double-blind, placebo-controlled, multicenter study of a ginger extract in the management of chemotherapy-induced nausea and vomiting (CINV) in patients receiving high-dose cisplatin. Ann. Oncol. 2017, 28, 2547–2551. [Google Scholar] [CrossRef] [PubMed]




| Author (Year) | Cohort Allocation | Study Design | Type of Disease/Symptom | Intervention | Comparator | Duration | Blind | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | M/F | Dosage | Number | M/F | Dosage | ||||||
| Marx et al. (2017) [17] | Australia | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 24 | 8/16 | 300 mg of ginger extract (5% gingerol)/cap, 4 capsules/day | 27 | 11/16 | 300 mg of placebo/cap, 4 capsules/day | 5 days | Double-blind |
| Sanaati et al. (2016) [18] | Iran | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 15 | 0/15 | 500 mg of powdered ginger/cap, 2 capsules/day and DMA regimen * | 15 | 0/15 | DMA regimen * | 5 days before and 5 days after chemotherapy | Double-blind |
| Iran | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 15 | 0/15 | 500 mg of powdered ginger/cap, 2 capsules/day and DMA regimen * | 15 | 0/15 | 500 mg of powdered chamomile/cap, 2 capsules/day and DMA regimen * | 5 days before and 5 days after chemotherapy | Double-blind | |
| Thamlikitkul et al. (2016) [19] | Thailand | Crossover randomized controlled trial | Chemotherapy-induced nausea and vomiting | Second cycle: 19; Third cycle: 15 | Second cycle: 0/19 Third cycle: 0/15 | 500 mg of powdered ginger/cap, 2 capsules/day | Second cycle: 15; Third cycle: 19 | Second cycle: 0/15; Third cycle: 0/19 | 500 mg of placebo/cap, 2 capsules/day | 5 days at each second and third cycle of chemotherapy | Double-blind |
| Li et al. (2017) [20] | China | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 71 | 53/18 | 250 mg of powdered ginger (5% gingerols)/cap, 2 capsules/day, bid | 69 | 47/22 | 250 mg of corn starch/cap, 2 capsules/day, bid | 5 days | Double-blind |
| Ansari et al. (2016) [21] | Iran | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 57 | 0/57 | 250 mg of powdered ginger/cap, 4 capsules/day, bid | 62 | 0/62 | 250 mg of starch/cap, 4 capsules/day, bid | 3 days for each 3 cycles | Single-blind |
| Sharifzadeh et al. (2018) [4] | Iran | Randomized controlled trial | Nausea and vomiting because of pregnancy | 28 | 0/28 | 500 mg of ginger/cap, 2 capsules/day | 26 | 0/26 | 40 mg of vitamin B6/cap, 2 capsules/day | 4 days | Triple- blind |
| Iran | Randomized controlled trial | Nausea and vomiting because of pregnancy | 28 | 0/28 | 500 mg of ginger/cap, 2 capsules/day | 23 | 0/23 | 2 placebo capsules/day | 4 days | Triple-blind | |
| Matsumura et al. (2015) [22] | United States | Randomized controlled trial | Muscle damage and delayed onset muscle soreness | 10 | 5/5 | 4 g of powdered ginger in capsule(s)/day | 10 | 5/5 | 4 g of dextrose in capsule(s)/day | 5 days | Double-blind |
| Martins et al. (2018) [23] | Brazil | Randomized controlled trial | Migraine | 30 | 4/26 | 200 mg of ginger extract/cap, 2 capsules/dose + 100 mg of ketoprofen (i.v.) | 30 | 4/26 | 200 mg of cellulose/cap, 2 capsules/dose + 100 mg of ketoprofen (i.v.) | Single dose | Double-blind |
| Arzati et al. (2017) [24] | Iran | Randomized controlled trial | Type 2 diabetes mellitus | 25 | 9/16 | 500 mg of ginger/cap, 4 capsules/day | 25 | 7/18 | 500 mg of wheat flour/cap, 4 capsules/day | 10 weeks | Double-blind |
| Attari et al. (2016) [25] | Iran | Randomized controlled trial | Obesity | 39 | 0/39 | 1 g of powdered ginger/tab, 2 tablets/day | 31 | 0/31 | 1 g of corn starch and other excipients/tab, 2tablets/day | 12 weeks | Double-blind |
| Attari et al. (2015) [26] | Iran | Randomized controlled trial | Obesity management | 39 | 0/39 | 1 g of powdered ginger/tab, 2 tablets/day | 31 | 0/31 | 1 g of corn starch/tab, 2 tablets/day | 12 weeks | Double-blind |
| Mozaffari-Khosravi et al. (2016) [13] | Iran | Randomized controlled trial | Knee osteoarthritis | 50 | 3/47 | 500 mg of powdered ginger/cap, 2 capsules/day | 50 | 7/43 | 500 mg of starch/cap, 2 capsules/day | 3 months | Double-blind |
| Aryaeian et al. (2019) [27] | Iran | Randomized controlled trial | Active rheumatoid arthritis | 33 | 4/29 | 750 mg of powdered ginger/cap, 2 capsules/day | 30 | 3/27 | 750 mg of wheat flour/cap, 2 capsules/day | 12 weeks | Double-blind |
| Kashefi et al. (2015) [28] | Iran | Randomized controlled trial | Heavy menstrual bleeding | 43 (1st month); 41 (2nd month); 38 (3rd month) | 0/43 (1st month) 0/41 (2nd month) 0/38 (3rd month) | 250 mg of powdered ginger/cap, 3 capsules/day | 43 (1st month); 38 (2nd month); 33 (3rd month) | 0/43 (1st month) 0/38 (2nd month) 0/33 (3rd month) | 250 mg of lactose/cap, 3 capsules/day | From the day before menstrual bleeding to the 3rd day of the menstrual period | Double-blind |
| Paritakul et al. (2016) [29] | Thailand | Randomized controlled trial | Breast milk volume of postpartum women who delivered a term baby (≥37 weeks of gestation) | 15 | 0/15 | 500 mg of powdered ginger/cap, 2 capsules/day | 21 | 0/21 | 500 mg of corn starch/cap, 2 capsules/day | 7 days | Double-blind |
| Ryan et al. (2012) [30] | United States | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 134 | 12/122 | One ginger capsule (250 mg of ginger extract) + 2 placebo capsules, twice/day | 149 | 14/135 | Three placebo capsules, twice/day | 6 days | Double-blind |
| United States | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 141 | 19/122 | Two ginger capsules (250 mg of ginger extract) + 1 placebo capsule, twice/day | ||||||
| United States | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 152 | 10/142 | Three ginger capsules (250 mg of ginger extract), twice/day | ||||||
| Zick et al. (2008) [31] | United States | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 53 | 14/39 | 250 mg of dry ginger root extract/cap, 4 ginger capsules and 4 lactose capsules/day | 57 | 14/43 | 250 mg of lactose/cap, 8 capsules/day | 28 days | Double-blind |
| United States | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 52 | 12/40 | 250 mg of dry ginger root extract/cap, 8 capsules/day | ||||||
| Fahimi et al. (2010) [32] | Iran | Crossover randomized controlled trial | Chemotherapy-induced nausea and vomiting | 36 | 26/10 | 250 mg of powdered ginger/cap, 2 capsules/day | 36 | 26/10 | 250 mg of lactose/cap, 2 capsules/day | 3 days for each period | Double-blind |
| Yekta et al. (2012) [33] | Iran | Randomized controlled trial | Chemotherapy-induced nausea and vomiting | 40 | 0/40 | 250 mg of powdered ginger/cap, 4 capsules/day | 40 | 0/40 | 250 mg of starch/cap, 4 capsules/day | 6 days from three days before a chemotherapy session | Double-blind |
| Ensiyeh et al. (2009) [3] | Iran | Randomized controlled trial | Nausea and vomiting because of pregnancy | 35 | 0/35 | 500 mg of powdered ginger/cap, 2 capsules/day | 34 | 0/34 | 20 mg of vitamin B6/cap, 2 capsules/day | 4 days | Double-blind |
| Willetts et al. (2003) [34] | Australia | Randomized controlled trial | Nausea and vomiting because of pregnancy | 48 | 0/48 | 125 mg of ginger extract/cap, 4 capsules/day | 51 | 0/51 | 4 capsules/day, each capsule containing soya bean oil | 4 days | Double-blind |
| Vutyavanich et al. (2001) [35] | Thailand | Randomized controlled trial | Nausea and vomiting because of pregnancy | 32 | 0/32 | 250 mg of powdered ginger/cap, 4 capsules/day | 38 | 0/38 | 4 placebo capsules/day | 4 days | Double-blind |
| Fischer-Rasmussen et al. (1990) [36] | Denmark | Crossover randomized controlled trial | Nausea and vomiting because of pregnancy | 27 | 0/27 | 250 mg of powdered ginger root/cap, 4 capsules/day | 27 | 0/27 | 250 mg of lactose/cap, 4 capsules/day | Two periods of 4 days | Double-blind |
| Smith et al. (2004) [37] | Australia | Randomized controlled trial | Nausea and vomiting because of pregnancy | 145 | 0/145 | 350 mg of ginger/cap, 3 capsules/day, tid | 146 | 0/146 | 25 mg of vitamin B6/cap, 3 capsules/day, tid | 3 weeks | Double-blind |
| Biswas et al. (2011) [38] | India | Randomized controlled trial | Nausea and vomiting because of pregnancy | 34 | 0/34 | 150 mg of dried ginger extract/tab, 3 tablets/day | 29 | 0/29 | 10 mg of doxylamine + 10 mg of pyridoxine/tab, 2 or 3 tablets/day | 3 weeks | Single-blind |
| Firouzbakht et al. (2014) [39] | Iran | Randomized controlled trial | Nausea and vomiting because of pregnancy | 24 | 0/24 | 250 mg of powdered ginger/cap, 4 capsules/day | 35 | 0/35 | 40 mg of vitamin B6/cap, 4 capsules/day | 4 days | Double-blind |
| Iran | Randomized controlled trial | Nausea and vomiting because of pregnancy | 24 | 0/24 | 250 mg of powdered ginger/cap, 4 capsules/day | 28 | 0/28 | 40 mg of sugar/cap, 4 capsules/day | 4 days | Double-blind | |
| Arfeen et al. (1995) [40] | Australia | Randomized controlled trial | Postoperative nausea and vomiting | 36 | N/A | One capsule containing 500 mg of powdered ginger and one placebo capsule | 36 | N/A | Two placebo capsules | Single-dose | Double-blind |
| Australia | Randomized controlled trial | Postoperative nausea and vomiting | 36 | N/A | Two capsules containing 500 mg of powdered ginger | 36 | N/A | Two placebo capsules | Single-dose | Double-blind | |
| Eberhart et al. (2003) [41] | Germany | Randomized controlled trial | Postoperative nausea and vomiting | 59 | 0/59 | 100 mg of ginger extract/cap, 1 ginger capsule + 1 placebo capsule/dose | 59 | 0/59 | 2 placebo capsules/dose | Triple dose: before operation and 3 h and 6 h post operation | Double-blind |
| Germany | Randomized controlled trial | Postoperative nausea and vomiting | 57 | 0/57 | 100 mg of ginger extract/cap, 2 ginger capsules/dose | 59 | 0/59 | 2 placebo capsules/dose | Triple dose: before operation and 3 h and 6 h post operation | Double-blind | |
| Mandal et al. (2014) [42] | India | Randomized controlled trial | Postoperative nausea and vomiting | 50 | 42/8 | 0.5 g of powdered ginger/cap, 2 capsules/dose + 4 mg of ondansetron (i.v.) | 50 | 38/12 | 2 capsules of placebo/dose + 4 mg of ondansetron (i.v.) | 1 h before induction of general anesthesia | Double-blind |
| Ozgoli et al. (2009) [43] | Iran | Randomized controlled trial | Primary dysmenorrhea | 50 | 0/50 | 250 mg of powdered ginger/cap, 4 capsules/day | 50 | 0/50 | 250 mg of mefenamic acid/cap, 4 capsules/day | 3 days | Double-blind |
| Iran | Randomized controlled trial | Primary dysmenorrhea | 50 | 0/50 | 250 mg of powdered ginger/cap, 4 capsules/day | 50 | 0/50 | 400 mg of ibuprofen/cap, 4 capsules/day | 3 days | Double-blind | |
| Kashefi et al. (2013) [44] | Iran | Randomized controlled trial | Primary dysmenorrhea | 47 (1st month); 45 (2nd month) | 0/47 (1st month) 0/45 (2nd month) | 250 mg of powdered ginger/cap, 3 capsules/day | 54 (1st month); 53 (2nd month) | 0/54 (1st month); 0/53 (2nd month) | 220 mg of zinc sulfate/cap, 3 capsules/day | 4 days | N/A |
| Iran | Randomized controlled trial | Primary dysmenorrhea | 47 (1st month); 45 (2nd month) | 0/47 (1st month) 0/45 (2nd month) | 250 mg of powdered ginger/cap, 3 capsules/day | 45 (1st month); 42 (2nd month) | 0/45 (1st month); 0/42 (2nd month) | 220 mg of lactose/cap, 3 capsules/day | 4 days | N/A | |
| Rahnama et al. (2012) [45] | Iran | Randomized controlled trial | Primary dysmenorrhea | 59 | 0/59 | 500 mg of powdered ginger root/cap, 3 capsules/day | 59 | 0/59 | 500 mg of toast powder (placebo)/cap, 3 capsules/day | From two days before the onset of menstrual period to first three days of menstrual period | Double-blind |
| Iran | Randomized controlled trial | Primary dysmenorrhea | 59 | 0/59 | 500 mg of powdered ginger root/cap, 3 capsules/day | 46 | 0/46 | 500 mg of toast powder (placebo)/cap, 3 capsules/day | First three days of the menstrual period | Double-blind | |
| Black et al. (2010) [46] | United States | Crossover randomized controlled trial | Muscle pain, inflammation, and dysfunction induced by eccentric exercise | 27 | 12/15 | Six capsules containing 2 g of dried ginger extract with 250 mL of water and one tablespoon of olive oil. | 27 | 12/15 | Six capsules containing 2 g of flour with 250 mL of water and one tablespoon of olive oil. | Single-dose | Double-blind |
| Black et al. (2009) [47] | United States | Randomized controlled trial | Muscle pain caused by eccentric exercise | Raw ginger study: 17 | Raw ginger study: 3/14 | Raw ginger study: 2 g of raw ginger /day | Raw ginger study: 17 | Raw ginger study: 3/14 | Raw ginger study: 2 g of yellow cornflower/day | 11 days | Double-blind |
| United States | Randomized controlled trial | Muscle pain caused by eccentric exercise | Heat-treated ginger study: 20 | Heat-treated ginger study: 7/13 | Heat-treated ginger study: 2 g of heat-treated ginger/day | Heat-treated ginger study: 20 | Heat-treated ginger study: 7/13 | Heat-treated ginger study: 2 g of powdered brown sugar/day | 11 days | Double-blind | |
| Mahluji et al. (2013) [48] | Iran | Randomized controlled trial | Type 2 diabetes mellitus | 28 | N/A | 1 g of powdered ginger/tab, 2 tablets/day | 30 | N/A | 1 g of corn starch/tab, 2 tablets/day | 8 weeks | Double-blind |
| Khandouzi et al. (2013) [49] | Iran | Randomized controlled trial | Type 2 diabetes mellitus | 22 | 5/17 | 1 g of powdered ginger/cap, 2 capsules/day | 19 | 9/10 | Lactose (placebo) | 12 weeks | Double-blind |
| Phillips et al. (1992) [50] | United Kingdom | Crossover randomized controlled trial | Gastric emptying | 16 | N/A | 500 mg of powdered ginger/cap, 2 capsules/dose | 16 | N/A | 500 mg of lactose/cap, 2 capsules/dose | Single-dose | Double-blind |
| Jiang et al. (2013) [14] | United States | Randomized controlled trial | Normal risk for colorectal cancer | 14 | N/A | 250 mg of ginger extract/cap, 8 capsules/day | 16 | N/A | Placebo (lactose) | 28 days | Double-blind |
| United States | Randomized controlled trial | High risk for colorectal cancer | 10 | 4/6 | 250 mg of ginger extract/cap, 8 capsules/day | 10 | 3/7 | Placebo (lactose) | 28 days | Double-blind | |
| Citronberg et al. (2013) [51] | United States | Randomized controlled trial | Cell cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer | 10 | 4/6 | 250 mg of ginger extract powder (5% gingerols)/cap, 8 capsules/day, bid | 10 | 3/7 | 250 mg of lactose powder /cap, 8 capsules/day, bid | 28 days | Double-blind |
| Zick et al. (2011) [52] | United States | Randomized controlled trial | Eicosanoids level of patients with normal risk for colorectal cancer | 16 | N/A | 250 mg of dry ginger root extract/cap, 8 capsules/day | 17 | N/A | 250 mg of lactose/cap, 8 capsules/day | 28 days | Triple-blind |
| Zick et al. (2014) [53] | United States | Randomized controlled trial | Eicosanoids level of patients with increased risk for colorectal cancer | 10 | 4/6 | 250 mg of dry ginger root extract (5% gingerols)/cap, 8 capsules/day | 10 | 3/7 | 250 mg of lactose/cap, 8 capsules/day | 28 days | Double-blind |
| Tilburg et al. (2014) [54] | United States | Randomized controlled trial | Irritable bowel syndrome | 15 | N/A | 1 g of ginger in capsules (2.29 mg/g of gingerols and 6-shogaol) | 15 | N/A | Brown sugar in capsules | 28 days | Double-blind |
| United States | Randomized controlled trial | Irritable bowel syndrome | 15 | N/A | 2 g of ginger in capsules (2.29 mg/g of gingerols and 6-shogaol) | ||||||
| Wigler et al. (2003) [55] | Israel | Crossover randomized controlled trial | Symptomatic gonarthritis | Group 1 (ginger first): 14; Group 2 (placebo first): 15 | Group 1 (ginger first): 1/13 Group 2 (placebo first): 5/10 | 250 mg of ginger extract (10 mg of gingerol)/cap, 4 capsules/day | Group 2 (placebo first): 15; Group 1 (ginger first): 14 | Group 2 (placebo first): 5/10; Group1 (ginger first): 1/13 | Placebo capsules, 4 capsules/day | Two periods of 12 weeks | Double-blind |
| Author (Year) | Evaluation Outcome System | Main Result | Adverse Effect |
|---|---|---|---|
| Marx et al. (2017) [17] | FLIE-5DR questionnaire, RINVR | Compared with placebo, ginger supplementation therapy can improve the chemotherapy-induced nausea-related quality of life and relieve vomiting and fatigue caused by chemotherapy. | No |
| Sanaati et al. (2016) [18] | Effects of the groups on nausea and vomiting using the generalized estimating equations (GEE) model | Ginger treatment reduced the frequency of vomiting and nausea significantly. | No |
| Thamlikitkul et al. (2016) [19] | Nausea score ( by VAS), vomiting incidence, rate of rescue medication use, and incidence of chemotherapy dose reduction | This study indicated that taking 1g of ginger for five days from the first day of chemotherapy had no effect in reducing the nausea severity of breast cancer patients receiving Adriamycin and cyclophosphamide chemotherapy. | No |
| Li et al. (2017) [20] | Incidence and severity of CINV by the MASCC Antiemesis Tool | In lung cancer patients who received cisplatin regimen, taking ginger as an adjuvant drug for antiemetics was ineffective in reducing the incidence and severity of CINV. | No |
| Ansari et al. (2016) [21] | Episodes of vomiting and nausea severity by nausea and vomiting grading | There was no significant improvement in breast cancer patients receiving chemotherapy regime-induced CINV upon ginger treatment. Thus, additional study is needed to make a conclusion. | No |
| Sharifzadeh et al. (2018) [4] | Rhodes questionnaire 2 | In relieving moderate to mild nausea and vomiting caused by pregnancy, ginger groups had similar effects to vitamin B6 and were more effective than the placebo group. | No |
| Matsumura et al. (2015) [22] | Creatine kinase, lactate dehydrogenase, 1RM, muscle soreness (by VAS), Circumference, ROM-flexion, ROM-extension, skin temp—non-dominant arm, skin temp—dominant arm | This study showed that taking 4 g of ginger may promote the recovery of muscle strength after intense exercise but has no effect on indicators of muscle damage or delayed onset muscle soreness. | No |
| Martins et al. (2018) [23] | Four-point scale, faces pain scale, visual numeric scale, nausea, ordinal scale, photophobia, phonophobia, and treatment satisfaction | The ginger-treated group showed a significant effect in reducing migraine attacks. | No |
| Arzati et al. (2017) [24] | FBS, total cholesterol, TG, LDL-cholesterol, HDL-cholesterol, HbA1C | Ginger supplementation significantly lowered fasting blood sugar, the mean variation of HbA1C, and LDL/HDL ratio. | No |
| Attari et al. (2016) [25] | Glucose, leptin, resistin, adiponectin, insulin, HOMA-IR, QUICKI | A little beneficial effect of ginger powder supplementation was found regarding improving biochemical obesity indicator and weight loss. | No |
| Attari et al. (2015) [26] | Obesity-associated parameters 1 | Subjects with the AA, UCP1, and Trp64Trp genotypes of β3ADR had significantly decreased anthropometric measurements and total appetite scores compared with the placebo group, but other evaluation outcomes were not significant. | No |
| Mozaffari-Khosravi et al. (2016) [13] | Serum TNF-α, serum IL-1β | In knee osteoarthritis patients, serum TNF-αNF-m IL-1β was decreased in both groups, with a lower level in the ginger group than the placebo group. | No |
| Aryaeian et al. (2019) [27] | Disease activity score-28, Gene expression levels of PPARγ, FOXP3, T-bet, GATA3, RORγt, and NFκB | This study showed that ginger could improve the active rheumatoid arthritis, as FOXP3 gene expression significantly increased within the ginger group and between two groups, whereas T-bet and RORγt gene expression decreased significantly between the two groups. | Yes |
| Kashefi et al. (2015) [28] | Percentage by which the mean hemorrhage decreased (%) | Ginger treatment reduced menstrual blood loss significantly during three interventions. | No |
| Paritakul et al. (2016) [29] | Breast milk volume on day three, breast milk volume on day seven, and serum prolactin level | Ginger treatment significantly increased milk volume on the third day compared to the placebo group. However, no significant difference was found in the milk volume and serum prolactin levels on the seventh day between the ginger and placebo groups. | No |
| Ryan et al. (2012) [30] | Seven point semantic rating, 13 item symptom inventory, and functional assessment of chronic illness therapy general | In adult cancer patients, a daily dose of 0.5–1.0 g of ginger was helpful in relieving the severity of acute chemotherapy-induced nausea | Yes |
| Zick et al. (2008) [31] | Prevalence and severity of delayed nausea and vomiting (by Morrow Assessment of Nausea and Emesis questionnaire) | Taking ginger as a reduction of CINV was insufficient, and there was no additional benefit for reducing the severity of acute and delayed CINV. | No |
| Fahimi et al. (2010) [32] | Prevalence, severity, and duration of acute and delayed nausea and vomiting (by Morrow Assessment of Nausea and Emesis) | Ginger treatment showed no effect in reducing the prevalence, severity, and duration of both acute and delayed nausea and vomiting | No |
| Yekta et al. (2012) [33] | Self-made, two-part self-reporting instrument (number of vomiting, use of other antiemetics, side effects) | The ginger treatment group decreased vomiting at anticipatory, acute, and delayed phases of patients who received chemotherapy. | Yes |
| Ensiyeh et al. (2009) [3] | Nausea scores, average number of vomiting episodes, follow-up visit, a five-point Likert scale | In early pregnancy, ginger intake had a stronger effect on relieving the severity of nausea than vitamin B6 intake. However, it had no significant difference in decreasing the number of vomiting episodes. | No |
| Willetts et al. (2003) [34] | Rhodes index of nausea, vomiting, and retching | Regarding nausea experience and retching, the ginger group was significantly lower than the placebo group of pregnancy-induced nausea. | No |
| Vutyavanich et al. (2001) [35] | Nausea (VAS score), number of vomiting episodes, and symptoms assessed by Likert scales | Nausea and vomiting induced in pregnancy could be relieved by ginger. | No |
| Fischer-Rasmussen et al. (1990) [36] | The severity of hyperemesis (by degree of nausea, vomiting, and weight loss) | Ginger treatment showed significantly greater relief on hyperemesis than a placebo. | No |
| Smith et al. (2004) [37] | Incidence of nausea, dry retching, and vomiting (by Rhodes index of nausea and vomiting form) and health status (by MOS 36 short form health survey) | Pregnant women with nausea, dry retching, vomiting can use ginger in the early stage of pregnancy to relieve the severity of symptoms as effectively as vitamin B6 | No |
| Biswas et al. (2011) [38] | The severity of dysmenorrhea, nausea, and vomiting (by VAS), average of nausea spells per day, vomiting episodes, average of nausea episodes, and average number of vomiting in last week | A ginger extract can be considered as safe therapy and effective alternative for the reduction of nausea and vomiting with no severe or serious adverse events. | No |
| Firouzbakht et al. (2014) [39] | The severity of nausea and the frequency of vomiting (by Likert scale and VAS) | After one week, the severity of nausea and vomiting was reduced dramatically in 60.6%, 42.7% and 61% of the ginger, placebo, and B6 groups, respectively. | Yes |
| Arfeen et al. (1995) [40] | The incidence of PONV and the distribution of nausea score | 0.5 or 1.0 g of ginger showed no efficacy on the incidence by postoperative nausea and vomiting. | No |
| Eberhart et al. (2003) [41] | The incidence rate of PONV, nausea, vomiting, and rescue antiemetics | There was no reduction in nausea, vomiting and the demand for antiemetic rescue treatment in three groups. | Yes |
| Mandal et al. (2014) [42] | Episodes of nausea, retching, vomiting and rescue antiemetic (by the score of Bellville), and severity of PONV (by VAS) | Ginger combined with ondansetron may be more helpful in controlled PONV than ondansetron alone. | No |
| Ozgoli et al. (2009) [43] | Self-administered questionnaire | When alleviating pain in women with primary dysmenorrhea, ginger was comparable to mefenamic acid and ibuprofen. | No |
| Kashefi et al. (2013) [44] | PVAS | There were differences in pain after administration in the ginger and zinc sulfate groups, and when compared with placebo groups, it was shown to be effective in both groups. | Yes |
| Rahnama et al. (2012) [45] | Pain (by VAS), duration of pain | There were significant differences in the severity of pain between the two groups, but only ‘protocol 1’ showed a significant difference in the duration of pain between two groups. | No |
| Black et al. (2010) [46] | Elbow range of motion, arm volume, VAS score, and metabolic rate | A single 2 g dose of ginger did not attenuate eccentric exercise-induced muscle pain, inflammation or dysfunction at 45 min after ingestion. However, ginger may attenuate the day-to-day progression of muscle pain. | No |
| Black et al. (2009) [47] | Arm muscle pain intensity muscle soreness (mm) 24-post, range of motion, Arm volume, Isometric force, △ROM (mean percent change in range of motion, %), △Volume (percent change in arm volume, %), △Force (percent change in isometric force, %), PGE2 (pg/mL), and ratings of perceived exertion | Taking raw and heat-treated ginger helped to reduce muscle pain following exercise-induced muscle injury. | No |
| Mahluji et al. (2013) [48] | Serum FBG, HbA1c, insulin, HOMA, QUICKI, TG, TC, LDL-C, HDL-C | Ginger supplementation had a significant effect in reducing the levels of insulin, LDL-C, TG, and HOMA, and it increased the QUICKI index compared to the control group. | Yes |
| Khandouzi et al. (2013) [49] | FBS, HbA1c, ApoB, Apo A-I, ApoB/Apo A-I, MDA | Ginger supplementation may help reduce FBS, HbA1c, ApoB, Apo A-I, ApoB/Apo A-I, MDA levels, as compare to the placebo and the baseline groups. | No |
| Phillips et al. (1992) [50] | Paracetamol concentration (time to peak and time to the first detection) | The oral ingestion of 1 g of ginger simultaneously with paracetamol did not affect the rate of absorption of paracetamol. Therefore, the study revealed that ginger had no better effect on gastric motility. | No |
| Jiang et al. (2013) [14] | cyclooxygenase-1 and 15-hydroxyprostaglandin protein levels | In the high risk of CRC participants, the colonic cyclooxygenase-1 protein level significantly decreased in the ginger group. On the other hand, 15-hydroxyprostaglandin was unchanged. There is no significant difference in average risk for CRC between the ginger and placebo groups. | No |
| Citronberg et al. (2013) [51] | Bax, Bcl-2, p21, hTERT, MIB-1, Bax/Bcl-2 Ratio, Bax/hTERT Ratio, Bax/MIB-1 Ratio, p21/hTERT Ratio, p21/MIB-1 Ratio, cell cycle score (w/MIB-1), and cell cycle score (w/hTERT) | Two grams of ginger extract may help in reducing the proliferation of normal-appearing colorectal epithelium, as well as increased apoptosis and differentiation relative to proliferation—especially in the differentiation zone of crypts | Yes |
| Zick et al. (2011) [52] | PGE2, 13-hydroxy-octadecadienoic acid, and 5-, 12-, and 15 hydroxyeicosatetraenoic acid | Ginger treatment may help to reduce eicosanoid levels by inhibiting synthesis from arachidonic acid. Additionally, ginger is considered to be safe for people with a high risk of colorectal cancer. | No |
| Zick et al. (2014) [53] | PGE2, LTB4, 13-hydroxy-octadecadienoic acids, and 5-, 12-, and 15-hydroxy-eicosatetraenoic acid | Treating root extraction with ginger for people at high risk for CRC for 28 days significantly decreased risk in the normal colonic mucosa of arachidonic acid and significantly increased LTB4, but other eicosanoids were ineffective. | Yes |
| Tilburg et al. (2014) [54] | Irritable bowel syndrome severity scale and adequate relief rating scale | In treating irritable bowel syndrome, ginger may not be a proper choice because the result of study could not suggest evidence for the better performance of the ginger treatment. | Yes |
| Wigler et al. (2003) [55] | VAS of pain on movement and handicap | After the crossover (three months), the ginger treatment group showed a significantly higher effect compared to the placebo group. | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients 2020, 12, 157. https://doi.org/10.3390/nu12010157
Anh NH, Kim SJ, Long NP, Min JE, Yoon YC, Lee EG, Kim M, Kim TJ, Yang YY, Son EY, et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients. 2020; 12(1):157. https://doi.org/10.3390/nu12010157
Chicago/Turabian StyleAnh, Nguyen Hoang, Sun Jo Kim, Nguyen Phuoc Long, Jung Eun Min, Young Cheol Yoon, Eun Goo Lee, Mina Kim, Tae Joon Kim, Yoon Young Yang, Eui Young Son, and et al. 2020. "Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials" Nutrients 12, no. 1: 157. https://doi.org/10.3390/nu12010157
APA StyleAnh, N. H., Kim, S. J., Long, N. P., Min, J. E., Yoon, Y. C., Lee, E. G., Kim, M., Kim, T. J., Yang, Y. Y., Son, E. Y., Yoon, S. J., Diem, N. C., Kim, H. M., & Kwon, S. W. (2020). Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients, 12(1), 157. https://doi.org/10.3390/nu12010157