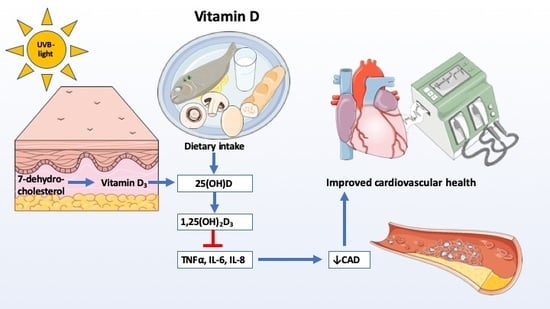
Potential Beneficial Effects of Vitamin D in Coronary Artery Disease
Abstract
:1. Introduction
2. Literature Search and Investigation
3. Coronary Artery Disease
3.1. Pathophysiology of Atherosclerosis
3.2. CAD Symptoms
3.3. CAD Prognosis
3.4. Diagnosis of CAD
3.5. Current Treatment Options of CAD
- Percutaneous coronary intervention (PCI): The PCI procedure is an effective strategy for revascularization in CAD patients with both acute and stable forms. The intervention is performed by inserting a guidewire catheter into the femoral or radial artery. The guidewire is guided to the coronary artery, where the thrombosis is located. Here, a balloon is inflated, and, for example, a metallic stent might be inserted in order to prevent reinfarction. Stents can either be bare metal or drug-eluting (everolimus, zotarolimus, etc.) to minimize restenosis [17,40]. In principle, PCI is the preferred procedure in patients with ST-segment elevation myocardial infarction (STEMI) within 12 h of symptom onset [41]. In addition, patients with non-ST-segment elevation myocardial infarction (NSTEMI) might be offered PCI within 48 h of symptom onset if no relevant comorbidity is present.
- Coronary artery bypass grafting (CABG): The CAGB procedure is considered more invasive compared to PCI. The procedure includes bypassing stenosed coronary arteries [42]. Thus, vein or artery grafts are used to anastomose occluded vessels. According to the SYNTAX study, the CABG strategy is preferable in more complex multivessel occlusions [43].
4. Vitamin D
4.1. Vitamin D Metabolism
4.2. Cardiovascular Effects of Vitamin D
4.2.1. Vitamin D and Essential Hypertension
4.2.2. Association between Serum Vitamin D and Myocardial Injury
4.2.3. Impact of Vitamin D on Cardiac Function after MI
4.2.4. Possible Mechanisms behind Vitamin D Effects on CAD
4.3. Vitamin D Supplementation and CAD
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, H.; Diamond, J.; Vieco, A.; Chaudhuri, S.; Shinnar, E.; Cromer, S.; Perel, P.; Mensah, G.A.; Narula, J.; Johnson, C.O.; et al. Global atlas of cardiovascular disease 2000–2016: The path to prevention and control. Glob. Heart 2018, 13, 143–163. [Google Scholar] [CrossRef]
- Fioranelli, M.; Bottaccioli, A.G.; Bottaccioli, F.; Bianchi, M.; Rovesti, M.; Roccia, M.G. Stress and inflammation in coronary artery disease: A review psychoneuroendocrineimmunology-based. Front. Immunol. 2018, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
- Christodoulidis, G.; Vittorio, T.J.; Fudim, M.; Lerakis, S.; Kosmas, C.E. Inflammation in coronary artery disease. Cardiol. Rev. 2014, 22, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Padro, T.; Vilahur, G.; Badimon, L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb. Haemost. 2012, 108, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Suades, R.; Arderiu, G.; Pena, E.; Chiva-Blanch, G.; Padro, T. Microvesicles in atherosclerosis and angiogenesis: From bench to bedside and reverse. Front. Cardiovasc. Med. 2017, 4, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slomka, A.; Piekus, A.; Kowalewski, M.; Pawliszak, W.; Anisimowicz, L.; Zekanowska, E. Assessment of the procoagulant activity of microparticles and the protein z system in patients undergoing off-pump coronary artery bypass surgery. Angiology 2018, 69, 347–357. [Google Scholar] [CrossRef]
- Hjerte. Available online: http://hjerteforeningen.dk/alt-om-dit-hjerte/hjertetal/hjertetaldk/ (accessed on 22 November 2019).
- Rai, V.; Agrawal, D.K. Role of vitamin D in cardiovascular diseases. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1039–1059. [Google Scholar] [CrossRef]
- Mendis, S.; Thygesen, K.; Kuulasmaa, K.; Giampaoli, S.; Mahonen, M.; Ngu Blackett, K.; Lisheng, L. World health organization definition of myocardial infarction: 2008-09 revision. Int. J. Epidemiol. 2011, 40, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef]
- George, J. Pathophysiology of coronary artery disease. In Interventional Cardiology Imaging: An Essential Guide; Abbas, A.E., Ed.; Springer: London, UK, 2015; pp. 29–46. [Google Scholar]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Cochain, C.; Zernecke, A. Macrophages in vascular inflammation and atherosclerosis. Pflüg. Arch. 2017, 469, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, A.; Cavallino, C.; Veia, A.; Bacchini, S.; Rosso, R.; Facchini, M.; Secco, G.G.; Lupi, A.; Nardi, F.; Rametta, F.; et al. Pathophysiology of atherosclerotic plaque development. Cardiovasc. Hematol. Agents Med. Chem. 2015, 13, 10–13. [Google Scholar] [CrossRef]
- Gistera, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2019. [Google Scholar] [CrossRef]
- Oeing, C.U.; Tschope, C.; Pieske, B. [The new ESC Guidelines for acute and chronic heart failure 2016]. Herz 2016, 41, 655–663. [Google Scholar] [CrossRef]
- Albert, N.M.; Lewis, C. Recognizing and managing asymptomatic left ventricular dysfunction after myocardial infarction. Crit. Care Nurse 2008, 28, 20–37, quiz 38. [Google Scholar]
- Philippides, G.J. Managing the post-myocardial infarction patient with asymptomatic left ventricular dysfunction. Cardiology 2006, 105, 95–107. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Bakker, A.J.; Gorgels, J.P.; van Vlies, B.; Koelemay, M.J.; Smits, R.; Tijssen, J.G.; Haagen, F.D. Contribution of creatine kinase mb mass concentration at admission to early diagnosis of acute myocardial infarction. Br. Heart J. 1994, 72, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Rumbinaite, E.; Zaliaduonyte-Peksiene, D.; Viezelis, M.; Ceponiene, I.; Lapinskas, T.; Zvirblyte, R.; Vencloviene, J.; Morkunaite, K.; Bielinis, A.; Slapikas, R.; et al. Dobutamine-stress echocardiography speckle-tracking imaging in the assessment of hemodynamic significance of coronary artery stenosis in patients with moderate and high probability of coronary artery disease. Medicina (Kaunas.) 2016, 52, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.P.; Ripley, D.P.; Berry, C.; McCann, G.P.; Plein, S.; Bucciarelli-Ducci, C.; Dall’Armellina, E.; Prasad, A.; Bijsterveld, P.; Foley, J.R.; et al. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or nice guidelines on subsequent unnecessary angiography rates: The ce-marc 2 randomized clinical trial. JAMA 2016, 316, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Gotschy, A.; Niemann, M.; Kozerke, S.; Luscher, T.F.; Manka, R. Cardiovascular magnetic resonance for the assessment of coronary artery disease. Int. J. Cardiol. 2015, 193, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.D.; Nayak, M.K. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr. Pharm. Des. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Scherwitz, L.W.; Billings, J.H.; Brown, S.E.; Gould, K.L.; Merritt, T.A.; Sparler, S.; Armstrong, W.T.; Ports, T.A.; Kirkeeide, R.L.; et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998, 280, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, S.R.; Wanstall, J.C. Beta-1 and beta-2 adrenoceptor-mediated responses in preparations of pulmonary artery and aorta from young and aged rats. J. Pharmacol. Exp. Ther. 1984, 228, 733–738. [Google Scholar] [PubMed]
- Patrono, C.; Morais, J.; Baigent, C.; Collet, J.P.; Fitzgerald, D.; Halvorsen, S.; Rocca, B.; Siegbahn, A.; Storey, R.F.; Vilahur, G. Antiplatelet agents for the treatment and prevention of coronary atherothrombosis. J. Am. Coll. Cardiol. 2017, 70, 1760–1776. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Tarkin, J.M.; Kaski, J.C. Vasodilator therapy: Nitrates and nicorandil. Cardiovasc. Drugs Ther. 2016, 30, 367–378. [Google Scholar] [CrossRef]
- Iachini Bellisarii, F.; Radico, F.; Muscente, F.; Horowitz, J.; De Caterina, R. Nitrates and other nitric oxide donors in cardiology: Current positioning and perspectives. Cardiovasc. Drugs Ther. 2012, 26, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.J.; Vaughan, D.E. Angiotensin-converting enzyme inhibitors. Circulation 1998, 97, 1411–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.K.; Griendling, K.K. Angiotensin ii cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, G. How cav1.2-bound verapamil blocks ca(2+) influx into cardiomyocyte: Atomic level views. Pharmacol. Res. 2019, 139, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Rayner-Hartley, E.; Sedlak, T. Ranolazine: A contemporary review. J. Am. Heart. Assoc. 2016, 5, e003196. [Google Scholar] [CrossRef] [Green Version]
- Fisch, A.S.; Perry, C.G.; Stephens, S.H.; Horenstein, R.B.; Shuldiner, A.R. Pharmacogenomics of anti-platelet and anti-coagulation therapy. Curr. Cardiol. Rep. 2013, 15, 381. [Google Scholar] [CrossRef] [Green Version]
- Favaloro, R.G.; Effler, D.B.; Cheanvechai, C.; Quint, R.A.; Sones, F.M., Jr. Acute coronary insufficiency (impending myocardial infarction and myocardial infarction): Surgical treatment by the saphenous vein graft technique. Am. J. Cardiol. 1971, 28, 598–607. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blomstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; Fernandez-Aviles, F.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef]
- Melly, L.; Torregrossa, G.; Lee, T.; Jansens, J.L.; Puskas, J.D. Fifty years of coronary artery bypass grafting. J. Thorac. Dis. 2018, 10, 1960–1967. [Google Scholar] [CrossRef] [Green Version]
- Mohr, F.W.; Morice, M.C.; Kappetein, A.P.; Feldman, T.E.; Stahle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R., Jr.; Morel, M.A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical syntax trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- Deluca, H.F. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, I.; Evans, I.M.; Larkins, R.G. Vitamin D. Clin. Endocrinol. (Oxf.) 1977, 6, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Patel, R.S.; Tangpricha, V.; Quyyumi, A.A. Vitamin D and cardiovascular disease: Is the evidence solid? Eur. Heart J. 2013, 34, 3691–3698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- IOM (Institute of Medicine). Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American association of clinical endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr. Pract. 2016, 22, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Thacher, T.D.; Clarke, B.L. Vitamin D insufficiency. Mayo Clin. Proc. 2011, 86, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Mosekilde, L.; Nielsen, R.; Larsen, E.R.; Moosgaard, B.; Heickendorff, L. [Vitamin D deficiency. Definition and prevalence in denmark]. Ugeskr Laeger 2005, 167, 29–33. [Google Scholar]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.R.; Bianchi, M.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. MANAGEMENT OF ENDOCRINE DISEASE: Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency; a position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, 23–54. [Google Scholar] [CrossRef] [Green Version]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Lockau, L.; Atkinson, S.A. Vitamin D’s role in health and disease: How does the present inform our understanding of the past? Int. J. Paleopathol. 2018, 23, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Marcocci, C.; Zucchi, R. Vitamin D status and cardiovascular outcome. J. Endocrinol. Investig. 2019, 42, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Swier, V.J.; Boosani, C.S.; Radwan, M.M.; Agrawal, D.K. Vitamin D deficiency accelerates coronary artery disease progression in swine. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1651–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.; Pan, W.; Kong, J.; Zheng, W.; Szeto, F.L.; Wong, K.E.; Cohen, R.; Klopot, A.; Zhang, Z.; Li, Y.C. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J. Biol. Chem. 2007, 282, 29821–29830. [Google Scholar] [CrossRef] [Green Version]
- Al-Ishaq, R.K.; Kubatka, P.; Brozmanova, M.; Gazdikova, K.; Caprnda, M.; Busselberg, D. Health implication of vitamin D on the cardiovascular and the renal system. Arch. Physiol. Biochem. 2019, 1–15. [Google Scholar] [CrossRef]
- Pilz, S.; Verheyen, N.; Grubler, M.R.; Tomaschitz, A.; Marz, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Legarth, C.; Grimm, D.; Wehland, M.; Bauer, J.; Kruger, M. The impact of vitamin D in the treatment of essential hypertension. Int. J. Mol. Sci. 2018, 19, 455. [Google Scholar] [CrossRef] [Green Version]
- Martins, D.; Wolf, M.; Pan, D.; Zadshir, A.; Tareen, N.; Thadhani, R.; Felsenfeld, A.; Levine, B.; Mehrotra, R.; Norris, K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the united states: Data from the third national health and nutrition examination survey. Arch. Intern. Med. 2007, 167, 1159–1165. [Google Scholar] [CrossRef] [Green Version]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the third national health and nutrition examination survey. Am. J. Hypertens. 2007, 20, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Chevli, P.A.; Li, Y.; Soliman, E.Z. Vitamin D deficiency and electrocardiographic subclinical myocardial injury: Results from national health and nutrition examination survey-iii. Clin. Cardiol. 2018, 41, 1468–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, V.L.; Harris, T. The third national health and nutrition examination survey: Contributing data on aging and health. Gerontologist 1994, 34, 486–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rautaharju, P.M.; Warren, J.W.; Jain, U.; Wolf, H.K.; Nielsen, C.L. Cardiac infarction injury score: An electrocardiographic coding scheme for ischemic heart disease. Circulation 1981, 64, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Verdoia, M.; Schaffer, A.; Sartori, C.; Barbieri, L.; Cassetti, E.; Marino, P.; Galasso, G.; De Luca, G. Vitamin D deficiency is independently associated with the extent of coronary artery disease. Eur. J. Clin. Investig. 2014, 44, 634–642. [Google Scholar] [CrossRef]
- Le, T.Y.L.; Ogawa, M.; Kizana, E.; Gunton, J.E.; Chong, J.J.H. Vitamin D improves cardiac function after myocardial infarction through modulation of resident cardiac progenitor cells. Heart Lung Circ. 2018, 27, 967–975. [Google Scholar] [CrossRef]
- Owen, M.K.; Noblet, J.N.; Sassoon, D.J.; Conteh, A.M.; Goodwill, A.G.; Tune, J.D. Perivascular adipose tissue and coronary vascular disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1643–1649. [Google Scholar] [CrossRef] [Green Version]
- Leonard, A.; Rahman, A.; Fazal, F. Importins alpha and beta signaling mediates endothelial cell inflammation and barrier disruption. Cell. Signal. 2018, 44, 103–117. [Google Scholar] [CrossRef]
- Sun, X.; Icli, B.; Wara, A.K.; Belkin, N.; He, S.; Kobzik, L.; Hunninghake, G.M.; Vera, M.P.; Blackwell, T.S.; Baron, R.M.; et al. Microrna-181b regulates nf-kappab-mediated vascular inflammation. J. Clin. Investig. 2012, 122, 1973–1990. [Google Scholar] [CrossRef]
- Boisvert, W.A.; Curtiss, L.K.; Terkeltaub, R.A. Interleukin-8 and its receptor cxcr2 in atherosclerosis. Immunol. Res. 2000, 21, 129–137. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The role of cytokines in the development of atherosclerosis. Biochemistry (Mosc.) 2016, 81, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.T.; Camargo, C.A., Jr. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: A randomized clinical trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hin, H.; Tomson, J.; Newman, C.; Kurien, R.; Lay, M.; Cox, J.; Sayer, J.; Hill, M.; Emberson, J.; Armitage, J.; et al. Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos. Int. 2017, 28, 841–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibert, E.; Lehmann, U.; Riedel, A.; Ulrich, C.; Hirche, F.; Brandsch, C.; Dierkes, J.; Girndt, M.; Stangl, G.I. Vitamin D3 supplementation does not modify cardiovascular risk profile of adults with inadequate vitamin D status. Eur. J. Nutr. 2017, 56, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Bassuk, S.S.; Lee, I.M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; Macfadyen, J.G.; Danielson, E.; Lin, J.; et al. The vitamin D and omega-3 trial (vital): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials 2012, 33, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Aslanabadi, N.; Jafaripor, I.; Sadeghi, S.; Hamishehkar, H.; Ghaffari, S.; Toluey, M.; Azizi, H.; Entezari-Maleki, T. Effect of vitamin D in the prevention of myocardial injury following elective percutaneous coronary intervention: A pilot randomized clinical trial. J. Clin. Pharmacol. 2018, 58, 144–151. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Menon, R.K.; Sharp, S.J.; Mannan, N.; Timms, P.M.; Martineau, A.R.; Rickard, A.P.; Boucher, B.J.; Chowdhury, T.A.; Griffiths, C.J.; et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: Results of a randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2016, 18, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Wang, T.; Zhu, S.; Li, L. Effects of vitamin D supplementation as an adjuvant therapy in coronary artery disease patients. Scand. Cardiovasc. J. 2016, 50, 9–16. [Google Scholar] [CrossRef]
- Shaseb, E.; Tohidi, M.; Abbasinazari, M.; Khalili, D.; Talasaz, A.H.; Omrani, H.; Hadaegh, F. The effect of a single dose of vitamin D on glycemic status and C-reactive protein levels in type 2 diabetic patients with ischemic heart disease: A randomized clinical trial. Acta Diabetol. 2016, 53, 575–582. [Google Scholar] [CrossRef]
- Dalan, R.; Liew, H.; Assam, P.N.; Chan, E.S.; Siddiqui, F.J.; Tan, A.W.; Chew, D.E.; Boehm, B.O.; Leow, M.K. A randomised controlled trial evaluating the impact of targeted vitamin D supplementation on endothelial function in type 2 diabetes mellitus: The dimension trial. Diabetes Vasc. Dis. Res. 2016, 13, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Sokol, S.I.; Srinivas, V.; Crandall, J.P.; Kim, M.; Tellides, G.; Lebastchi, A.H.; Yu, Y.; Gupta, A.K.; Alderman, M.H. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc. Med. 2012, 17, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnson, Y.; Itzhaky, D.; Mosseri, M.; Barak, V.; Tzur, B.; Agmon-Levin, N.; Amital, H. Vitamin D inflammatory cytokines and coronary events: A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Dove, F.J.; Khan, F.; Lang, C.C.; Belch, J.J.; Struthers, A.D. Effects of vitamin D supplementation on markers of vascular function after myocardial infarction—a randomised controlled trial. Int. J. Cardiol. 2013, 167, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Allison, M.A.; Carr, J.J.; Langer, R.D.; Cochrane, B.B.; Hendrix, S.L.; Hsia, J.; Hunt, J.R.; Lewis, C.E.; Margolis, K.L.; et al. Calcium/vitamin D supplementation and coronary artery calcification in the women’s health initiative. Menopause 2010, 17, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrokhian, A.; Raygan, F.; Bahmani, F.; Talari, H.R.; Esfandiari, R.; Esmaillzadeh, A.; Asemi, Z. Long-term vitamin D supplementation affects metabolic status in vitamin D-deficient type 2 diabetic patients with coronary artery disease. J. Nutr. 2017, 147, 384–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 50–55. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Valenti, M.T.; Del Forno, F.; Caneva, E.; Pietrobelli, A. Vitamin D: Daily vs. Monthly use in children and elderly—what is going on? Nutrients 2017, 9, 652. [Google Scholar] [CrossRef] [Green Version]
- Tanakol, R.; Gul, N.; Uzum, A.K.; Aral, F. Calcitriol treatment in patients with low vitamin D levels. Arch. Osteoporos. 2018, 13, 114. [Google Scholar] [CrossRef]
- Song, Q.; Sergeev, I. Calcium and vitamin D in obesity. Nutr. Res. Rev. 2012, 25, 130–141. [Google Scholar] [CrossRef]
- Sergeev, I.N. Vitamin D—Cellular Ca2+ link to obesity and diabetes. J. Steroid Biochem. Mol. Biol. 2016, 164, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Sergeev, I.N. Vitamin D-mediated apoptosis in cancer and obesity. Horm. Mol. Biol. Clin. Investig. 2014, 20, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D supplementation and cardiovascular disease risks in more than 83000 individuals in 21 randomized clinical trials: A meta-analysis. JAMA Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]



| Classification | Characteristics |
|---|---|
| Typical angina | Constricting sensation in front of chest or shoulder, neck, jaw or arm. Symptoms relieved by nitrates or rest ≤ 5 min. Triggered by physical exertion. |
| Atypical angina | Meets only two of the characteristics above. |
| Non-anginal chest pain | Meets none or just one of the characteristics above. |
| Procedure | Explanation |
|---|---|
| Electrocardiogram (ECG) [21] | ECG plays a key role in the initial diagnosis in patients presenting with angina symptoms. A resting 12-lead ECG may reveal abnormalities that support the diagnosis of MI or myocardial ischemia. ST-segment deviations (depression/elevation) may visualize myocardial injury. |
| Biochemical tests [17,22] | This procedure may include blood samples with a lipid profile, fasting glucose and glycated hemoglobin (HbA1c), full blood count and plasma creatinine. Furthermore, it is essential to measure myocardial injury markers such as troponin I, troponin T and creatine kinase myocardial band (CK-MB). |
| Echocardiography [23] | An echocardiography might be performed as a stress test under physical exercise or under concomitant administration of medication such as dipyridamole or dobutamine. This procedure might reveal areas in the left ventricle (LV) with wall abnormalities or hypocontractility. |
| Cardiovascular magnetic resonance (CMR) [24] | This procedure utilizes electromagnetic waves for the imaging of heart and coronary vessels. This technique can be used to assess myocardial viability after MI [25]. |
| Coronary catheterization and angiography [11] | As recommended by the 2019 European Society of Cardiology (ESC) guidelines, this technique is now used in cases of inconclusive non-invasive tests and for patients with a high clinical likelihood and severe symptoms refractory to medical therapy or high event risk. A catheter is guided from a peripheral artery to the coronary artery. Subsequently, a contrast medium is injected, and coronary arteries are visualized by X-ray. |
| Class | Indications | Mechanism | Drugs |
|---|---|---|---|
| Beta-adrenoceptor antagonists [28] | arrhythmia, hypertension, post-MI, angina pectoris, CAD | Act through the blockade of beta-adrenoceptors in cardiac muscle cells and vascular SMCs. Lead to decreased heart rate and cardiac output (CO). Secondarily, antagonism of β1-adrenoceptors will cause relaxation of vascular smooth muscle cells, induce vasodilation and lower total periphery resistance (TPR). | Metoprolol Propranolol Carvedilol Atenolol Nebivolol |
| Acetylsalicylic acid (ASA) [29] | acute MI, CAD, prevent re-thrombosis | Irreversible inactivation of cyclooxygenase (COX-1, -2) enzymes. This inhibition promotes a blockade of thromboxane synthesis, which decreases platelet activation. | Aspirin |
| Statins [30,31] | hyperlipidemia, dyslipidemia, post-MI | Inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase enzyme (HMG-CoA reductase), which catalyzes the transformation of HMG to mevalonic acid, thereby reducing intracellular cholesterol concentrations, which leads to the upregulation of LDL surface receptors. Eventually, plasma concentrations of LDL and total cholesterol fall with statin treatment. | Simvastatin Rosuvastatin Atorvastatin Pravastatin |
| Nitrates [32,33] | angina pectoris, CAD | Promote the release of nitric oxide (NO) in smooth muscle cells in blood vessels. High intracellular NO concentrations activate guanylyl cyclase (GC). Eventually, this results in decreased intracellular calcium concentrations. This causes dilatation of the coronary arteries, afterload reduction and increased angina threshold. | Glyceryl nitrate Isosorbide dinitrate Isosorbide mononitrate |
| Angiotensin-converting enzyme inhibitors (ACE-inhibitors) [34,35] | hypertension, LV heart failure (HF), heart failure post- MI, angina pectoris | The enzyme ACE hydrolyzes angiotensin I to the active form angiotensin II (Ang II). Effects carried out by Ang II are decreased by the inhibition of ACE. This promotes vasoconstriction, upregulation of aldosterone secretion in the kidneys and fibrosis in cardiac cells, resulting in vasodilation, reduced TPR and reduced preload and afterload. | Enalapril Captopril Ramipril Trandolapril |
| Calcium channel blockers [36] | hypertension, angina pectoris | Calcium channel blockers act through the blockade of L-type Ca2+ channels in vascular smooth muscle cells. The inhibition of voltage-gated calcium channels results in the decreased release of Ca2+ into the cytoplasm. This causes vasodilatation and a fall in TPR and blood pressure. Moreover, non-dihydropyridine derivatives (such as diltiazem and verapamil) have a direct effect on cardiac muscle cells. This leads to subsequent negative inotropy and negative chronotropy. | Diltiazem Verapamil Felodipin Amlodipin Lercadipin |
| Anti-anginal [37] | chronic angina pectoris | Acts through the inhibition of late influx sodium channels (INa) in cardiomyocytes. Thus, calcium overload is attenuated by reduced sodium/calcium exchange in cardiac myocytes. Subsequently, the oxygen demand is reduced and cardiac output improves. | Ranolazine |
| Anti-platelet medications [38] | MI, stroke, UAP, stent patients | The mechanism of action is carried out via the blockade of the Gi-protein coupled P2Y12 receptor. This causes inhibition of the intracellular PI3K pathway and diminished platelet activation and aggregation. | Clopidogrel Ticagrelor Prasugrel |
| Health organization | Optimal | Insufficiency | Deficiency | Reference |
|---|---|---|---|---|
| Endocrine Society | ≥30 ng/mL (75 nmol/L), preferred range: 40–60 ng/mL | 21–29 ng/mL | ≤20 ng/mL (50 nmol/L) | [48] |
| The Institute of Medicine (Health and Medicine Division of the National Academies) | ≥20 ng/mL | 12–20 ng/mL | <12 ng/mL | [49] |
| The American Association of Clinical Endocrinologists | 30–50 ng/mL | 20–29 ng/mL | <20 ng/mL | [50] |
| Mayo Clinic | >20 ng/mL | 11–20 ng/mL | ≤10 ng/mL | [51] |
| Danish Health Authority | 20–64 ng/mL (50–160 nmol/L) | 10–20 ng/mL (25–50 nmol/L) | <10 ng/mL (25 nmol/L) | [52] |
| ECTS working group | ≥20 ng/mL (50 nmol/L) | N/A | <20 ng/mL (50 nmol/L) Severe: <12 ng/mL (30 nmol/L) | [53] |
| Title, Study Identifier | Design | Endpoints | Objective | Conclusions |
|---|---|---|---|---|
| Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. (ViDA) [75] ACTRN12611000402943. | Randomized Double-blinded Placebo-controlled n = 5110 Median follow-up = 3.3 years. | Cardiovascular disease (CVD) events Mortality MI | To investigate if monthly supplementation of cholecalciferol (100,000 IU) could prevent CVD events in vitamin D insufficient patients. | Intervention with vitamin D supplementation significantly raises mean serum 25(OH)D-levels, when compared to placebo. However, this study found no beneficial effects of cholecalciferol supplementation on CVD risk or mortality. |
| Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. [76] EudraCT number: 2011-005763-24a | Randomized Placebo-controlled Parallel group assignment Double-blinded n = 305 | BP PWV Cholesterol Heart rate | To evaluate the effects of daily supplementation for one year of cholecalciferol (4000 IU or 2000 IU) on disease risk and biochemical markers in healthy elderly people. | Vitamin D supplementation restores serum levels 25(OH)D, but no significant changes in CVD risk factors, arterial stiffness, blood lipids or blood pressure were observed after the intervention. |
| Vitamin D3 supplementation does not modify cardiovascular risk profile of adults with inadequate vitamin D status. [77] NCT01711905 | Randomized Placebo-controlled Double-blinded n = 106 | BP Blood lipids Heart rate Renin | To investigate if daily supplementation for 12 weeks of cholecalciferol (800 IU/day) could decrease blood pressure, heart rate and other cardiovascular risk markers in healthy participants. | Vitamin D did not improve cardiovascular risk markers in this study, even though serum 25(OH)D levels were restored. |
| Vitamin D supplements and prevention of cancer and cardiovascular disease. (ViTAL) [78] NCT01169259 | Randomized Placebo-controlled Double-blinded n = 25,871 | Mortality CVD events MI | To elucidate if daily supplementation for an average of 5.3 years of cholecalciferol (2000 IU/day) could reduce the risk of major cardiovascular events and invasive cancer in a normal population. | Vitamin D supplementation did not improve cardiac health, even though serum 25(OH)D levels were restored. Furthermore, no reduction in mortality was observed. The risk of MI was not significantly reduced in the intervention group (hazard ratio = 0.96 (95% CI: 0.78–1.19). |
| Effect of vitamin D in the prevention of myocardial injury following elective percutaneous coronary intervention (PCI): a pilot randomized clinical trial. [79] IRCT201402078307N6 | Randomized Placebo-controlled Double-blinded n = 99 | Cardiac injury CK-MB cTnl | To investigate if a 300,000 IU dose of cholecalciferol given before PCI could prevent myocardial injury. | No significant changes in cardiac injury markers were noted. However, the biomarkers CK-MB and cTnI were non-significantly improved (p > 0.05). The cardiac parameter hsCRP was statistically altered in favor of vitamin D when the two groups were compared at baseline and 24 h later. (−2.1 and −3: p = 0.045). No incidence of major adverse cardiovascular events (MACE) occurred in the study groups, thereby effects on incident CAD events could not be evaluated. |
| Effects of vitamin D2 or D3 supplementation on glycemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double-blind placebo-controlled trial. [80] EudraCT 2009-011264-11 | Randomized Placebo-controlled Double-blinded n = 340 | HbA1c PWV hsCRP Blood lipids | To investigate whether monthly supplementation of either ergocalciferol (100,000 IU/month) or cholecalciferol (100,000 IU/month) orally administered for four months could improve cardiometabolic parameters in patients with high risk of diabetes type 2. | Monthly supplementation restored blood serum levels of 25(OH)D. However, this short-term treatment did not significantly improve the overall cardiometabolic parameters. The only significant findings were improvements in pulse wave velocity (PWV). PWV: ergocalciferol; −0.68 m/s (95% CI: −1.31, −0.05) and cholecalciferol; −0.73 m/s (95% CI: −1.42, −0.03). |
| Effects of vitamin D supplementation as an adjuvant therapy in coronary artery disease patients. [81] ChiCTR-TRC-13003958 | Randomized Placebo-controlled Double-blinded n = 90 | Coronary artery disease (SYNTAX score) Blood lipids BP HbA1c | To examine if daily supplementation with calcitriol (0.5 μg/day) for six months in stable CAD patients could enhance the SYNTAX score and cardiometabolic parameters. | Mean serum levels of 25(OH)D were restored in the intervention group. After six months, the interventional group had a significantly decreased SYNTAX score of −3.9 (95% CI: −6.6 to −0.7, p < 0.001). Furthermore, hsCRP decreased by −0.07 mg/dl (95% CI: −0.13 to −0.02, p < 0.001). However, the other secondary outcomes did not change significantly. The authors concluded that calcitriol supplementation is beneficial in CAD. |
| The effect of a single dose of vitamin D on glycemic status and C-reactive protein levels in type 2 diabetic patients with ischemic heart disease: a randomized clinical trial. [82] ACTRN12614000529640. | Randomized Placebo-controlled Double-blinded n = 95 | hsCRP HbA1c Fasting blood sugar (FBS) | Whether a single dose of cholecalciferol (300,000 IU, i.m.) could improve glycemic status in type 2 diabetes patients. | Vitamin D status was restored after the intervention when compared to placebo. However, this study found no significant changes in vascular inflammation in the intervention group vs. placebo (hsCRP +1236.95 ng/mL (standard error 842.1, p = 0.3)). However, HbA1c was reduced by 0.48% (p = 0.04) in the vitamin D supplementation group. It was concluded that cholecalciferol improves glycemic status but fails to improve vascular inflammation. |
| A randomized controlled trial (RCT) evaluating the impact of targeted vitamin D supplementation on endothelial function in type 2 diabetes mellitus: The DIMENSION trial. [83] NCT01741181 | Randomized Placebo-controlled Double-blinded n = 31 | hsCRP Von Willebrand factor E-selectin Reactive hyperemia index | Whether daily supplementation for 16 weeks with cholecalciferol (either 2000 IU/day or 4000 IU/day) could improve vascular biomarkers and the reactive hyperemia index in patients with type 2 diabetes. | Vitamin D status was significantly improved in the intervention arm. After multivariate regression analysis, this study found no significant changes in parameters of endothelial function (p > 0.05). |
| The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. [84] NCT01570309 | Randomized Placebo-controlled Double-blinded n = 90 | hsCRP BPPro-inflamma-tory cytokines Adhesion molecules Endothelial function | To elucidate if a weekly supplementation with ergocalciferol (50,000 IU/week) for 12 weeks in patients with CAD could enhance endothelial function and vascular inflammation. | Mean average serum 25(OH)D was significantly raised in the intervention group vs. placebo. No significant improvements were demonstrated in markers of vascular inflammation (p = 0.79). Furthermore, blood pressure and all markers of endothelial function were not significantly impacted (p > 0.05). Hence, this study failed to find any cardiovascular benefits of weekly vitamin D supplementation. |
| Vitamin D inflammatory cytokines and coronary events: a comprehensive review [85]. | Randomized Placebo-controlled Double-blinded n = 50 | Adhesion molecules (VCAM-1, ICAM-1, E-selectin, VEGF) Pro-inflammatory cytokines (CRP, IL-6, IL-8, TNF-α) | To examine the cardiovascular effects of daily oral supplementation with cholecalciferol (4000 IU/day) for five days in patients presenting with acute MI and undergoing PCI. | Five days of cholecalciferol supplementation led to insignificant alterations in mean average serum 25(OH)D levels (p = 0.14). CRP underwent a significantly smaller increase in the intervention group vs. placebo (108.6% vs. 361%, p = 0.03). Likewise, a significant reduction in IL-6 (−31.6%; p = 0.05) was observed. VCAM-1 was reduced by 3.3% compared to a 23% increase in controls (p = 0.03). ICAM-1, E-selectin and VEGF were all insignificantly changed (p > 0.15). Thus, some inflammatory and adhesion markers were affected, while others were insignificantly impacted by vitamin D supplementation. |
| Effects of vitamin D supplementation on markers of vascular function after myocardial infarction—a randomized controlled trial. [86] EuDRACT ref: 2009-010367-17 | Randomized Placebo-controlled Double-blinded n = 75 | Blood pressure Cholesterol Reactive hyperemia index CRP Von Willebrand factor TNF-α E-selectinB-type natriuretic peptide | To investigate if two high-doses of orally administered cholecalciferol (100,000 IU) could improve cardiovascular markers in patients with a prior history of MI. | Serum 25(OH)D levels were only modestly improved after five days. No significant differences were observed in the reactive hyperemia index, systolic BP, diastolic BP or cholesterol levels. The only marker showing a significant change was CRP (−1.3 vs. +2.0 mg/L, p = 0.03). Thus, high-dose vitamin D supplementation insignificantly improved cardiovascular markers after MI. |
| Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. [87] NCT00000611 | Randomized Placebo-controlled Double-blinded n = 754 All women | Coronary artery calcium (CAC) score | To assess the vascular effect of daily supplementation with calcium (1000 mg/day) + cholecalciferol (400 IU/day) for an average of seven years in women. The objective was to evaluate if supplementation could alter the plaque burden in the intervention group. | Serum vitamin D status at baseline and after intervention was not obtained in this study. The study found no significant changes in CAC score. Adjusting the multivariate odds ratio (OR) for incremental CAC scores failed to demonstrate a significant reduction in plaque burden (p > 0.05). |
| Long-term vitamin D supplementation affects metabolic status in vitamin D-deficient type 2 diabetic patients with coronary artery disease. [88] IRCT201510315623N56 | Randomized Placebo-controlled Double-blinded n = 60 | Fasting blood glucose hsCRP Plasma NO Serum insulin | Sought to examine if supplementation with 50,000 IU cholecalciferol every second week for six months could improve vascular inflammation and glycemic markers. Participants were type 2 diabetic patients with CAD. | Vitamin D status was markedly improved in the intervention arm during follow-up. The results showed a significant attenuation in vascular inflammation (including hsCRP and plasma NO reductions, p < 0.05) and improved glycemic status in diabetic patients supplemented with vitamin D for six months. |
| The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo- controlled trial. [89] IRCT2017073033941N4 | Randomized Placebo-controlled Double-blinded n = 60 | Mental health parameters hsCRP Plasma NO Glycemic control HDL cholesterol | To investigate if combined vitamin D (50,000 IU) + probiotic supplementation every second week for 12 weeks could be beneficial regarding cardiovascular parameters in type 2 diabetic patients with CAD. | Combined vitamin D and probiotic supplementation restored serum 25(OH)D status (p < 0.05). Vitamin D + probiotics seemed to significantly improve vascular inflammation (lower hsCRP) and glycemic markers. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legarth, C.; Grimm, D.; Krüger, M.; Infanger, M.; Wehland, M. Potential Beneficial Effects of Vitamin D in Coronary Artery Disease. Nutrients 2020, 12, 99. https://doi.org/10.3390/nu12010099
Legarth C, Grimm D, Krüger M, Infanger M, Wehland M. Potential Beneficial Effects of Vitamin D in Coronary Artery Disease. Nutrients. 2020; 12(1):99. https://doi.org/10.3390/nu12010099
Chicago/Turabian StyleLegarth, Christian, Daniela Grimm, Marcus Krüger, Manfred Infanger, and Markus Wehland. 2020. "Potential Beneficial Effects of Vitamin D in Coronary Artery Disease" Nutrients 12, no. 1: 99. https://doi.org/10.3390/nu12010099
APA StyleLegarth, C., Grimm, D., Krüger, M., Infanger, M., & Wehland, M. (2020). Potential Beneficial Effects of Vitamin D in Coronary Artery Disease. Nutrients, 12(1), 99. https://doi.org/10.3390/nu12010099