Unique Triterpenoid of Jujube Root Protects Cisplatin-induced Damage in Kidney Epithelial LLC-PK1 Cells via Autophagy Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 3DC2ME from Z. jujube
2.2. Cell Culture and Reagents
2.3. Protective Effect Against Cisplatin-Induced Nephrotoxicity in LLC-PK1 Kidney Cells
2.4. Analysis of Autophagosomes
2.5. Western Blot Analysis
2.6. Image-Based Cytometric Assay
2.7. Statistical Analysis
3. Results
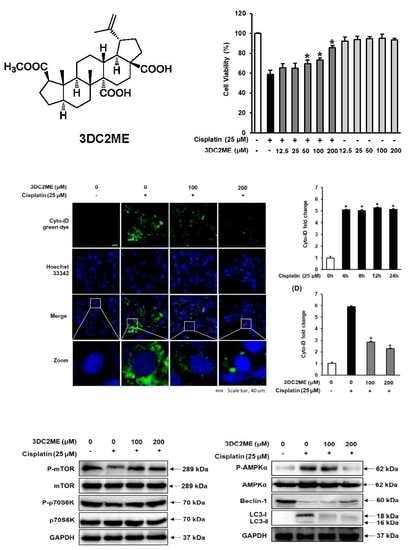
3.1. Protective Effects of 3DC2ME Against Cisplatin-Induced Kidney Cell Damage
3.2. Effect of 3DC2ME on Autophagic Vacuoles in LLC-PK1 Cells
3.3. Effect of Cisplatin on Protein Expressions of AMPK/mTOR-Dependent Signaling Pathway in LLC-PK1 Cells
3.4. Effect of 3DC2ME on Protein Expressions of AMPK/mTOR-Dependent Signaling Pathway in LLC-PK1 Cells
3.5. Effects of Autophagy Inhibitor 3-MA and 3DC2ME on Expression of Autophagy-Related Protein and Apoptosis-Related Proteins in LLC-PK1 Cells
3.6. Effects of Autophagy Inhibitor 3-MA and 3DC2ME Against Cisplatin-Induced Apoptosis in LLC-PK1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swarnalatha, Y.; Prabavathy, D. Quantification of Bcl-2/Bax genes in A549 Lung Cancer Cell Lines Treated with Heptamethoxy Flavones. Asian J. Pharm. 2018, 12, S667–S673. [Google Scholar]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Venkataraman, B.; Kurdi, A.; Mahgoub, E.; Sadek, B.; Rajesh, M. Plant-Derived Agents for Counteracting Cisplatin-Induced Nephrotoxicity. Oxid. Med. Cell Longev. 2016, 2016, 4320374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundararajan, R.; Bharampuram, A.; Koduru, R. A Review on Phyto-Constituents for Nephroprotective Activity. Pharmacophore 2014, 5, 160–182. [Google Scholar]
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Pareek, S. Nutritional composition of jujube fruit. Emir. J. Food Agric. 2013, 25, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Hovanet, M.V.; Oprea, E.; Ancuceanu, R.V.; Dutu, L.E.; Budura, E.A.; Seremet, O.; Ancu, I.; Morosan, E. Wound Healing Properties of Ziziphus jujuba Mill. leaves. Rom. Biotech. Lett. 2016, 21, 11842–11849. [Google Scholar]
- Preeti, K.; Tripathi, S. Ziziphus jujuba: A phytopharmacological review. Int. J. Res. Dev. Life Sci. 2014, 3, 959–966. [Google Scholar]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.X.; Tsim, K.W.K. A Review of Dietary Ziziphus jujuba Fruit (Jujube): Developing Health Food Supplements for Brain Protection. Evid. Based Complement. Alternat. Med. 2017, 2017, 3019568. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; An, X.; Lee, B.H.; Lee, J.S.; Heo, H.J.; Kim, T.; Ahn, J.-W.; Kim, D.-O. Protective effects of bioactive phenolics from jujube (Ziziphus jujuba) seeds against H2O2-induced oxidative stress in neuronal PC-12 cells. Food Sci. Biotechnol. 2015, 24, 2219–2227. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Jiang, X.; Sun, Y.; Zhao, Z.; Li, S. Protective Effect of Flavonoids from Ziziphus jujuba cv. Jinsixiaozao against Acetaminophen-Induced Liver Injury by Inhibiting Oxidative Stress and Inflammation in Mice. Molecules 2017, 22, 1781. [Google Scholar] [CrossRef]
- Preethi, J.; Vennila, K.; Penislusshiyan, S.; Velvizhi, S. Hepatoprotective and Antioxidant Role of Ziziphus jujuba Leaves on Paracetamol Induced Hepatic Damage in Rats. J. Dis. Med. Plants 2016, 2, 1–10. [Google Scholar]
- Hovanet, M.V.; Ancuceanu, R.V.; Dinu, M.; Oprea, E.; Budura, E.A.; Negres, S.; Velescu, B.S.; Dutu, L.E.; Anghel, I.A.; Ancu, I.; et al. Toxicity and Anti-Inflammatory Activity of Ziziphus Jujuba Mill. Leaves. Farmacia 2016, 64, 802–808. [Google Scholar]
- Ebrahimi, S.; Mollaei, H.; Hoshyar, R. Ziziphus Jujube: A review study of its anticancer effects in various tumor models invitro and invivo. Cell Mol. Biol. 2017, 63, 122–127. [Google Scholar] [CrossRef]
- Tahergorabi, Z.; Abedini, M.R.; Mitra, M.; Fard, M.H.; Beydokhti, H. “Ziziphus jujuba”: A red fruit with promising anticancer activities. Pharmacol. Res. 2015, 9, 99. [Google Scholar]
- Pitchaiah, G.K.S.; Prabhakar, J.; Hari Sravanth Reddy, H.; Kumar, A. Renoprotective activity of ziziphus jujuba fruit extract in gentamicin-induced nephrotoxic rats. IJPT 2005, 6, 194–198. [Google Scholar]
- Awad, D.S.; Ali, R.M.; Mhaidat, N.M.; Shotar, A.M. Zizyphus jujuba protects against ibuprofen-induced nephrotoxicity in rats. Pharm. Biol. 2014, 52, 182–186. [Google Scholar] [CrossRef]
- Yue, Y.; Wu, S.; Zhang, H.; Zhang, X.; Niu, Y.; Cao, X.; Huang, F.; Ding, H. Characterization and hepatoprotective effect of polysaccharides from Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou sarcocarp. Food Chem. Toxicol. 2014, 74, 76–84. [Google Scholar] [CrossRef]
- Hamedi, S.; Arian, A.A.; Farzaei, M.H. Gastroprotective effect of aqueous stem bark extract of Ziziphus jujuba L. against HCl/Ethanol-induced gastric mucosal injury in rats. J. Tradit. Chin. Med. 2015, 35, 666–670. [Google Scholar] [CrossRef]
- Alam, S.; Hussain, M.S.; Reddy, M.K.; Reddy, M.; Gupta, R.K. Antiulcer and Antioxidant Potential of Zizyphus jujuba Mill Root Extract In Aspirin And Ethanol Induced Gastric Ulcers. IJP 2016, 8, 287–293. [Google Scholar]
- Kubota, H.; Morii, R.; Kojima-Yuasa, A.; Huang, X.; Yano, Y.; Matsui-Yuasa, I. Effect of Zizyphus jujuba extract on the inhibition of adipogenesis in 3T3-L1 preadipocytes. Am. J. Chin. Med. 2009, 37, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Mesaik, A.M.; Poh, H.W.; Bin, O.Y.; Elawad, I.; Alsayed, B. In Vivo Anti-Inflammatory, Anti-Bacterial and Anti-Diarrhoeal Activity of Ziziphus Jujuba Fruit Extract. Open Access Maced. J. Med. Sci. 2018, 6, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Kawai, Y.; Gemba, M. Cisplatin-induced renal injury in LLC-PK1 cells. In Proceedings of the 6th World Congress on Alternatives & Animal Use in the Life Sciences, Tokyo, Japan, August 2007; pp. 453–456. [Google Scholar]
- Gunness, P.; Aleksa, K.; Kosuge, K.; Ito, S.; Koren, G. Comparison of the novel HK-2 human renal proximal tubular cell line with the standard LLC-PK1 cell line in studying drug-induced nephrotoxicity. Can. J. Physiol. Pharmacol. 2010, 88, 448–455. [Google Scholar] [CrossRef]
- Pfaller, W.; Gstraunthaler, G. Nephrotoxicity testing in vitro-what we know and what we need to know. Environ. Health Perspect. 1998, 106, 559–569. [Google Scholar]
- Perantoni, A.; Berman, J.J. Properties of Wilms’ tumor line (TuWi) and pig kidney line (LLC-PK 1) typical of normal kidney tubular epithelium. In Vitro 1979, 15, 446–454. [Google Scholar] [CrossRef]
- Inoue, K.; Kuwana, H.; Shimamura, Y.; Ogata, K.; Taniguchi, Y.; Kagawa, T.; Horino, T.; Takao, T.; Morita, T.; Sasaki, S.; et al. Cisplatin-induced macroautophagy occurs prior to apoptosis in proximal tubules in vivo. Clin. Exp. Nephrol. 2010, 14, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Xu, Q.; Liu, S.; Yu, B.; Liu, J.; Tang, J. Role of autophagy and its signaling pathways in ischemia/reperfusion injury. Am. J. Transl. Res. 2017, 9, 4470. [Google Scholar]
- Xing, J.J.; Hou, J.G.; Ma, Z.N.; Wang, Z.; Ren, S.; Wang, Y.P.; Liu, W.C.; Chen, C.; Li, W. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019, 52, e12627. [Google Scholar] [CrossRef]
- Kang, K.B.; Kim, J.W.; Oh, W.K.; Kim, J.; Sung, S.H. Cytotoxic Ceanothane- and Lupane-Type Triterpenoids from the Roots of Ziziphus jujuba. J. Nat. Prod. 2016, 79, 2364–2375. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, K.H.; Lee, W.Y.; Kim, C.E.; Sung, S.H.; Kang, K.B.; Kang, K.S. Multiple Targets of 3-Dehydroxyceanothetric Acid 2-Methyl Ester to Protect Against Cisplatin-Induced Cytotoxicity in Kidney Epithelial LLC-PK1 Cells. Molecules 2019, 24, 878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosohata, K. Role of oxidative stress in drug-induced kidney injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, H.; Kaminski, D.; Gangaraju, R.; Adebiyi, A. Cisplatin-induced oxidative stress stimulates renal Fas ligand shedding. Ren. Fail. 2018, 40, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, R.E.; Saleh, D.O.; Mansour, D.F. Cisplatin-Induced Nephrotoxicity in Rats: Modulatory Role of Simvastatin and Rosuvastatin against Apoptosis and Inflammation. JAPS 2018, 8, 43–50. [Google Scholar]
- Cummings, B.S.; Schnellmann, R.G. Cisplatin-induced renal cell apoptosis: Caspase 3-dependent and-independent pathways. J. Pharmacol. Exp. Ther. 2002, 302, 8–17. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Shah, S.V. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Kaushal, V.; Shah, S.V.; Kaushal, G.P. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am. J. Physiol. Ren. 2008, 294, F777–F787. [Google Scholar] [CrossRef] [Green Version]
- Havasi, A.; Dong, Z. Autophagy and Tubular Cell Death in the Kidney. Semin. Nephrol. 2016, 36, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Gu, L.; Chen, D.; Wu, W.; Liu, H.; Hu, H.; Wan, Y.; Sun, W. Rhein Inhibits Autophagy in Rat Renal Tubular Cells by Regulation of AMPK/mTOR Signaling. Sci. Rep. 2017, 7, 43790. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Kaushal, V.; Herzog, C.; Yang, C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy 2008, 4, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic, R.; Cvijanovic, O.; Pernjak-Pugel, E.; Skoda, M.; Mikelic, L.; Crncevic-Orlic, Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 2013, 62, 397–406. [Google Scholar] [CrossRef]
- Jia, H.; Yan, Y.; Liang, Z.; Tandra, N.; Zhang, B.; Wang, J.; Xu, W.; Qian, H. Autophagy: A new treatment strategy for MSC-based therapy in acute kidney injury (Review). Mol. Med. Rep. 2018, 17, 3439–3447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Gui, Y.; Ren, J.; Liu, X.; Feng, Y.; Zeng, Z.; He, W.; Yang, J.; Dai, C. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKα-regulated autophagy induction. Sci. Rep. 2016, 6, 23975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Chen, W.; Zou, Y.; Huang, H.; Pan, B.; Jin, S.; Huang, R.; Nie, S.; Kong, G. AMP-activated protein kinase regulates autophagic protection against cisplatin-induced tissue injury in the kidney. GMR 2015, 14, 12006–12015. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kang, K.B.; Kim, H.W.; Park, J.S.; Hwang, G.S.; Kang, K.S.; Choi, S.; Yamabe, N.; Kim, K.H. Unique Triterpenoid of Jujube Root Protects Cisplatin-induced Damage in Kidney Epithelial LLC-PK1 Cells via Autophagy Regulation. Nutrients 2020, 12, 677. https://doi.org/10.3390/nu12030677
Lee D, Kang KB, Kim HW, Park JS, Hwang GS, Kang KS, Choi S, Yamabe N, Kim KH. Unique Triterpenoid of Jujube Root Protects Cisplatin-induced Damage in Kidney Epithelial LLC-PK1 Cells via Autophagy Regulation. Nutrients. 2020; 12(3):677. https://doi.org/10.3390/nu12030677
Chicago/Turabian StyleLee, Dahae, Kyo Bin Kang, Hyun Woo Kim, Jung Sik Park, Gwi Seo Hwang, Ki Sung Kang, Sungyoul Choi, Noriko Yamabe, and Ki Hyun Kim. 2020. "Unique Triterpenoid of Jujube Root Protects Cisplatin-induced Damage in Kidney Epithelial LLC-PK1 Cells via Autophagy Regulation" Nutrients 12, no. 3: 677. https://doi.org/10.3390/nu12030677
APA StyleLee, D., Kang, K. B., Kim, H. W., Park, J. S., Hwang, G. S., Kang, K. S., Choi, S., Yamabe, N., & Kim, K. H. (2020). Unique Triterpenoid of Jujube Root Protects Cisplatin-induced Damage in Kidney Epithelial LLC-PK1 Cells via Autophagy Regulation. Nutrients, 12(3), 677. https://doi.org/10.3390/nu12030677