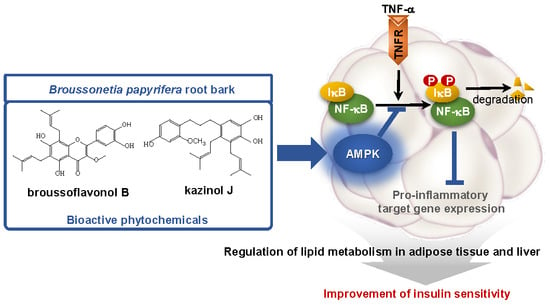
Broussonetia papyrifera Root Bark Extract Exhibits Anti-inflammatory Effects on Adipose Tissue and Improves Insulin Sensitivity Potentially Via AMPK Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of B. papyrifera Root Bark
2.3. Cell Culture and Adipocyte Differentiation
2.4. Reporter Gene Assay
2.5. Nitric Oxide Production and Cell Viability in Raw 264 Cells
2.6. Animal Experiment
2.7. Histological Analysis
2.8. Western Blot Analysis
2.9. Gene Expression
2.10. Statistical Analysis
3. Results
3.1. PRE Suppresses TNF-α-Induced NF-κB Activity
3.2. PRE Suppresses Pro-Inflammatory Gene Expression in Adipocytes
3.3. PRE Improves Obesity-Associated Systemic Glucose Tolerance
3.4. PRE Ameliorates Adipose Tissue Inflammation
3.5. PRE Ameliorates Hepatic Steatosis
3.6. Broussoflavonol B and Kazinol J are Bioactive Compounds of PRE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Invest. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Li, P.; Huh, J.Y.; Hwang, I.J.; Lu, M.; Kim, J.I.; Ham, M.; Talukdar, S.; Chen, A.; Lu, W.J.; et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 2011, 60, 2474–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrino. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 15, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Salt, I.P.; Palmer, T.M. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert. Opin. Investig. Drugs. 2012, 21, 1155–1167. [Google Scholar] [CrossRef]
- Bai, A.; Ma, A.G.; Yong, M.; Weiss, C.R.; Ma, Y.; Guan, Z.; Bernstein, C.N.; Peng, Z. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem. Pharmacol. 2010, 80, 1708–1717. [Google Scholar] [CrossRef]
- Nath, N.; Giri, S.; Prasad, R.; Salem, M.L.; Singh, A.K.; Singh, I. 5-Aminoimidazole-4-Carboxamide Ribonucleoside: A Novel Immunomodulator with Therapeutic Efficacy in Experimental Autoimmune Encephalomyelitis. J. Immunol. 2005, 175, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Myerburg, M.M.; J Darwin King, J.; Oyster, N.M.; Fitch, A.C.; Magill, A.; Baty, C.J.; Watkins, S.C.; Kolls, J.K.; Pilewski, J.M.; Hallows, K.R. AMPK Agonists Ameliorate Sodium and Fluid Transport and Inflammation in Cystic Fibrosis Airway Epithelial Cells. Am. J. Respir. Cell. Mol. Biol. 2010, 42, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Galic, S.; Fullerton, M.D.; Schertzer, J.D.; Sikkema, S.; Marcinko, K.; Walkley, C.R.; Izon, D.; Honeyman, J.; Chen, Z.P.; van Denderen, B.J.; et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Invest. 2011, 121, 12. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Kahn, B.B.; Shi, H.; Xue, B.Z. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010, 285, 19051–19059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhang, X.; Wang, H.; Guo, X.; Li, H.; Wang, Y.; Xu, X.; Tan, L.; Mashek, M.T.; Zhang, C.; et al. AMP-Activated Protein Kinase α1 Protects Against Diet-Induced Insulin Resistance and Obesity. Diabetes 2012, 12, 3114–3125. [Google Scholar]
- Ko, H.H.; Chang, W.L.; Lu, T.M. Antityrosinase and antioxidant effects of ent-kaurane diterpenes from leaves of Broussonetia papyrifera. J. Nat. Prod. 2008, 71, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.Q.; Wang, Y.H.; Du, G.H.; Liu, G.M.; Zhang, L.; CGebg, Y.X. Antioxidant lignans from the fruits of Broussonetia papyrifera. J. Nat. Prod. 2009, 72, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.W.; Chen, H.Y.; Wu, C.R.; Liao, P.M.; Lin, Y.T.; Hsieh, M.T.; Ching, H. Comparison with various parts of Broussonetia papyrifera as to the antinociceptive and anti-inflammatory activities in rodents. Biosci. Biotechnol. Biochem. 2008, 72, 2377–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.H.; Ahn, H.; Lee, H.J. Inhibition of nitric oxide production on LPS-activated macrophages by kazinol B from Broussonetia kazinoki. Fitoterapia 2003, 74, 350–354. [Google Scholar] [CrossRef]
- Lee, H.; Li, H.; Jeong, J.H.; Noh, M.; Ryu, J.H. Kazinol B from Broussonetia kazinoki improves insulin sensitivity via Akt and AMPK activation in 3T3-L1 adipocytes. Fitoterapia 2016, 112, 90–96. [Google Scholar] [CrossRef]
- Ryu, H.W.; Park, M.H.; Kwon, O.K.; Kim, D.Y.; Hwang, J.Y.; Jo, Y.H.; Ahn, K.S.; Hwang, B.Y.; Oh, S.R. Anti-inflammatory flavonoids from root bark of Broussonetia papyrifera in LPS-stimulated RAW264.7 cells. Bioorg. Chem. 2019, 92, 103233. [Google Scholar] [CrossRef]
- Park, J.Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Par, K.H.; Ryu, Y.B.; Lee, W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 504–512. [Google Scholar] [CrossRef] [Green Version]
- Ryu, H.W.; Lee, B.W.; Curtis-Long, M.J.; Jung, S.; Ryu, Y.B.; Lee, W.S.; Park, K.H. Polyphenols from Broussonetia papyrifera displaying potent alpha-glucosidase inhibition. J. Agric. Food Chem. 2010, 58, 202–208. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 2005, 115, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.C.; Wang, S.; Wu, Y.; Chen, R.Y.; Yu, D.Q. Five New Diprenylated Flavonols from the Leaves of Broussonetia kazinoki. J. Nat. Prod. 2001, 64, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Huang, B.K.; Qin, L.P. The genus Broussonetia: A review of its phytochemistry and pharmacology. Phytother. Res. 2012, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, H.; Ryu, J.H. Prenylated Polyphenols from Broussonetia kazinoki as Inhibitors of Nitric Oxide Production. Molecules 2018, 23, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 5-Monophosphate-Activated Protein Kinase Promotes Macrophage Polarization to an Anti-Inflammatory Functional Phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, Y.-J.; Zhang, X.; Wang, X.; Bao, B.; Qu, W.; Li, J. Luteolin reduces obesity-associated insulin resistance in mice by activating AMPKα1 signalling in adipose tissue macrophages. Diabetologia 2016, 59, 2219–2228. [Google Scholar] [CrossRef]
- Jeong, H.W.; Hsu, K.C.; Lee, J.W.; Ham, M.; Huh, J.Y.; Shin, H.J.; Kim, W.S.; Kim, J.B. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E955–E964. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF -κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell. Mol. Life Sci. 2010, 67, 3407–3423. [Google Scholar] [CrossRef] [Green Version]
- Palomer, X.; Álvarez-Guardia, D.; Rodríguez-Calvo, R.; Coll, T.; Laguna, J.C.; Davison, M.M.; Chan, T.O.; Feldman, A.M.; Vázquez-Carrera, M. TNF-α reduces PGC-1α expression through NF-κB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovas. Res. 2009, 81, 703–712. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.M.; Choi, S.S.; Park, M.H.; Jang, H.; Lee, Y.H.; Khim, K.W.; Oh, S.R.; Park, J.; Ryu, H.W.; Choi, J.H. Broussonetia papyrifera Root Bark Extract Exhibits Anti-inflammatory Effects on Adipose Tissue and Improves Insulin Sensitivity Potentially Via AMPK Activation. Nutrients 2020, 12, 773. https://doi.org/10.3390/nu12030773
Lee JM, Choi SS, Park MH, Jang H, Lee YH, Khim KW, Oh SR, Park J, Ryu HW, Choi JH. Broussonetia papyrifera Root Bark Extract Exhibits Anti-inflammatory Effects on Adipose Tissue and Improves Insulin Sensitivity Potentially Via AMPK Activation. Nutrients. 2020; 12(3):773. https://doi.org/10.3390/nu12030773
Chicago/Turabian StyleLee, Jae Min, Sun Sil Choi, Mi Hyeon Park, Hyunduk Jang, Yo Han Lee, Keon Woo Khim, Sei Ryang Oh, Jiyoung Park, Hyung Won Ryu, and Jang Hyun Choi. 2020. "Broussonetia papyrifera Root Bark Extract Exhibits Anti-inflammatory Effects on Adipose Tissue and Improves Insulin Sensitivity Potentially Via AMPK Activation" Nutrients 12, no. 3: 773. https://doi.org/10.3390/nu12030773
APA StyleLee, J. M., Choi, S. S., Park, M. H., Jang, H., Lee, Y. H., Khim, K. W., Oh, S. R., Park, J., Ryu, H. W., & Choi, J. H. (2020). Broussonetia papyrifera Root Bark Extract Exhibits Anti-inflammatory Effects on Adipose Tissue and Improves Insulin Sensitivity Potentially Via AMPK Activation. Nutrients, 12(3), 773. https://doi.org/10.3390/nu12030773