Parent and Provider Perspectives on the Imprecise Label of “Human Milk Fortifier” in the NICU
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Development
2.2. Survey Distribution
2.3. Analysis
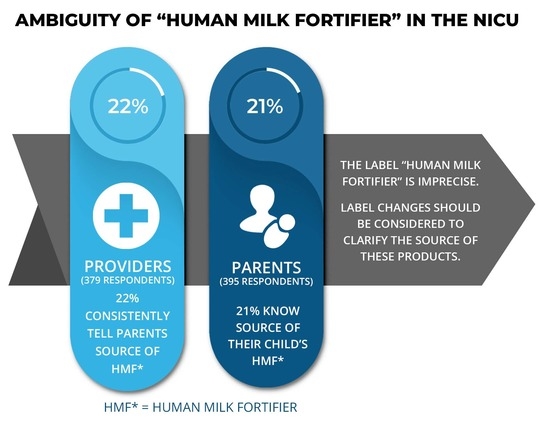
3. Results
3.1. Parent Survey
3.1.1. Demographics
3.1.2. Feeding Type
3.1.3. Necrotizing Enterocolitis
3.1.4. Adjectives Describing Mother’s Own Milk or Fortification
3.2. Provider Survey
Demographics
3.3. Human Milk Fortifier Information and Labeling
Qualitative Survey Data
“I wish I would have been told that the fortifier was cow’s-milk based. The name is way too confusing.”
“It should be very clear whether the fortifier is derived from cow’s-milk formula or breast milk. A label with “human milk” should only mean it’s FROM humans.”
“While in the NICU with my baby, I had to ask what HMF stood for and I only really understood what it was once we were home and I could see the bottle and read the ingredient label.”
“Until today, I had no understanding of what “fortifier” meant when nurses used that term. It’s disappointing how uninformed parents are in the NICU during such a vulnerable time.”
“The labeling of our cow’s milk-based fortifiers is misleading because it still has the label “human milk.”
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meinzen-Derr, J.; Poindexter, B.; Wrage, L.; Morrow, A.L.; Stoll, B.; Donovan, E.F. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J. Perinatol. 2009, 29, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.; Tonkin, E.; Damarell, R.; McPhee, A.; Suganuma, M.; Suganuma, H.; Middleton, P.; Makrides, M.; Collins, C. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2014, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.V.; Embleton, N.D.; Harding, J.E.; McGuire, W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst. Rev. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, S.A.; Bryan, M.H.; Anderson, G.H. Human milk feeding in premature infants: Protein, fat, and carbohydrate balances in the first two weeks of life. J. Pediatrics 1981, 99, 617–624. [Google Scholar] [CrossRef]
- Gadepalli, S.K.; Canvasser, J.; Eskenazi, Y.; Quinn, M.; Kim, J.H.; Gephart, S.M. Roles and Experiences of Parents in Necrotizing Enterocolitis: An International Survey of Parental Perspectives of Communication in the NICU. Adv. Neonatal Care 2017, 17, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.S.; Cooper, L.G.; Blaine, A.I.; Franck, L.S.; Howse, J.L.; Berns, S.D. Family Support and Family-Centered Care in the Neonatal Intensive Care Unit: Origins, Advances, Impact. Semin. Perinatol. 2011, 35, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineda, R.; Bender, J.; Hall, B.; Shabosky, L.; Annecca, A.; Smith, J. Parent participation in the neonatal intensive care unit: Predictors and relationships to neurobehavior and developmental outcomes. Early Hum. Dev. 2018, 117, 32–38. [Google Scholar] [CrossRef] [PubMed]

| Parent Cohort % (n) | |
|---|---|
| Age (years): | N = 345 |
| Under 18 | 0.5% (2) |
| 18–29 | 20% (68) |
| 30–39 | 57% (195) |
| 40–50 | 21% (74) |
| 50 Years or above | 1.5% (6) |
| Race/Ethnicity: | N = 358 |
| White | 85% (305) |
| Hispanic or Latino | 7% (24) |
| Black | 3% (11) |
| Asian/Asian Indian | 3% (12) |
| Other | 2% (6) |
| Formal Education: | N = 344 |
| High School Degree | 20% (69) |
| College Graduate | 45% (156) |
| Post-Graduate Degree | 30% (101) |
| Other | 5% (18) |
| Year Child was Born: | N = 395 |
| 2010 and Below | 14% (54) |
| 2011–2013 | 16% (62) |
| 2014–2016 | 25% (101) |
| 2017–2019 | 45% (177) |
| Child Gestational Age at Birth: | N = 395 |
| 22–24 weeks | 17% (65) |
| 25–28 weeks | 35% (137) |
| 29–33 weeks | 29% (115) |
| 34–36 weeks | 12% (49) |
| 37–42 weeks | 7% (29) |
| Provider Cohort | |
|---|---|
| N = 379 | |
| % (n) | |
| Position: | |
| Nurse | 52% (196) |
| Physician | 28% (108) |
| Neonatal Nurse Practitioner | 8% (29) |
| Registered Dietitian | 6% (21) |
| Fellow/Resident | 4% (15) |
| Physician Assistant | 1% (5) |
| Lactation Consultant | 1% (5) |
| Length of Providing NICU Care: | |
| More Than 20 Years | 27% (101) |
| 10–19 years | 31% (117) |
| 5–9 years | 25% (96) |
| 1–4 years | 15% (58) |
| Less Than a Year | 2% (7) |
| Race/Ethnicity: | |
| White | 80% (305) |
| Asian/Asian Indian | 8.5% (32) |
| Hispanic or Latino | 5% (18) |
| Black | 3% (11) |
| Other | 3.5% (13) |
| %(n) Choosing the Response * | Parent Survey:“In the NICU, What Did HMF Mean to You?” | Parent Survey:“Today, What Words Describe HMF?” | %(n) Choosing the Response * | Provider Survey:“What Does the Label HMF Mean to You?” |
|---|---|---|---|---|
| Cow’s milk-based product | 10% (35) | 22% (75) | Cow’s milk-based product | 52% (194) |
| Human milk-based product | 21% (71) | 49% (171) | Human milk-based product | 35% (133) |
| Vitamins and minerals | 27% (93) | 19% (67) | Concentrated formula | 12% (45) |
| Additional calorie source | 64% (221) | 44% (151) | Supplement in addition to human milk | 39% (146) |
| Not sure | 13% (44) | 14% (48) | Additive to human milk | 83% (313) |
| Other | 7% (24) | 4% (13) | Other | 2% (8) |
| Response | Parent (n = 344) | Provider (n = 377) |
|---|---|---|
| For cow’s milk-based fortifier | ||
| Cow’s milk-based fortifier | 73% (251) | 54% (203) |
| Concentrated formula | 20% (70) | 8% (31) |
| Human milk fortifier | 4% (13) | 27% (100) |
| Other* | 3% (10) | 11% (43) |
| For human milk-based fortifier | ||
| Human milk-based fortifier | 53% (183) | 59% (224) |
| Donor milk fortifier | 24% (83) | 19% (70) |
| Human milk fortifier | 20% (67) | 17% (66) |
| Other* | 3% (10) | 5% (19) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canvasser, J.; Hair, A.B.; Kim, J.H.; Taylor, S.N. Parent and Provider Perspectives on the Imprecise Label of “Human Milk Fortifier” in the NICU. Nutrients 2020, 12, 720. https://doi.org/10.3390/nu12030720
Canvasser J, Hair AB, Kim JH, Taylor SN. Parent and Provider Perspectives on the Imprecise Label of “Human Milk Fortifier” in the NICU. Nutrients. 2020; 12(3):720. https://doi.org/10.3390/nu12030720
Chicago/Turabian StyleCanvasser, Jennifer, Amy B. Hair, Jae H. Kim, and Sarah N. Taylor. 2020. "Parent and Provider Perspectives on the Imprecise Label of “Human Milk Fortifier” in the NICU" Nutrients 12, no. 3: 720. https://doi.org/10.3390/nu12030720
APA StyleCanvasser, J., Hair, A. B., Kim, J. H., & Taylor, S. N. (2020). Parent and Provider Perspectives on the Imprecise Label of “Human Milk Fortifier” in the NICU. Nutrients, 12(3), 720. https://doi.org/10.3390/nu12030720