Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
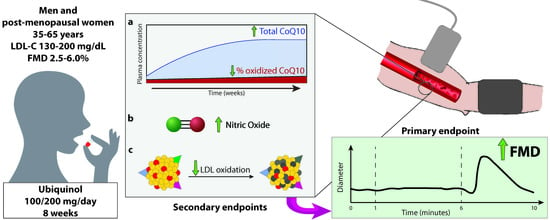
2.3. Primary and Secondary Endpoints
2.4. Laboratory Measurements
2.5. Assessment of Flow-Mediated Dilation
2.6. Determination of Plasma Coenzyme Q10 Levels
2.7. Determination of Serum Nitric Oxide Metabolites
2.8. LDL Oxidation Assay
2.9. Sample Size and Statistical Analysis
3. Results
3.1. Study Population
3.2. Flow-Mediated Dilation
3.3. Plasma Total and Oxidized Coenzyme Q10
3.4. Evaluation of Serum Nitric Oxide Metabolites
3.5. Evaluation of Plasma Oxidized LDL and LDL Susceptibility to Oxidation In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Prospective Studies, C.; Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef]
- Hu, G.; Qiao, Q.; Tuomilehto, J.; Balkau, B.; Borch-Johnsen, K.; Pyorala, K.; Group, D.S. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch. Intern. Med. 2004, 164, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Kit, B.K.; Kuklina, E.; Carroll, M.D.; Ostchega, Y.; Freedman, D.S.; Ogden, C.L. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015, 169, 272–279. [Google Scholar] [CrossRef] [Green Version]
- van den Oever, I.A.; Raterman, H.G.; Nurmohamed, M.T.; Simsek, S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediat. Inflamm. 2010, 2010, 792393. [Google Scholar] [CrossRef] [Green Version]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Invest. 2016, 126, 821–828. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Vignini, A.; Salvolini, E.; Nanetti, L.; Mazzanti, L.; Anna Rabini, R. Platelet-Derived NO in Subjects Affected by Type 2 Diabetes with and without Complications: Is there any Relationship with their Offspring? Exp. Clin. Endocrinol. Diabetes 2017, 125, 290–296. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [Green Version]
- Davies, P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pr. Cardiovasc. Med. 2009, 6, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijssen, D.H.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.A.; Kundt, G.; Lenschow, U.; Schuff-Werner, P.; Kienast, W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J. Am. Coll. Cardiol. 2006, 48, 1865–1870. [Google Scholar] [CrossRef] [Green Version]
- Heiss, C.; Jahn, S.; Taylor, M.; Real, W.M.; Angeli, F.S.; Wong, M.L.; Amabile, N.; Prasad, M.; Rassaf, T.; Ottaviani, J.I.; et al. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J. Am. Coll. Cardiol. 2010, 56, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Charakida, M.; Masi, S.; Luscher, T.F.; Kastelein, J.J.; Deanfield, J.E. Assessment of atherosclerosis: The role of flow-mediated dilatation. Eur. Heart J. 2010, 31, 2854–2861. [Google Scholar] [CrossRef]
- Greyling, A.; van Mil, A.C.; Zock, P.L.; Green, D.J.; Ghiadoni, L.; Thijssen, D.H.; on Flow, T.I. Adherence to guidelines strongly improves reproducibility of brachial artery flow-mediated dilation. Atherosclerosis 2016, 248, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Doring, F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors 2008, 32, 179–183. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Clinical aspects of coenzyme Q10: An update. Nutrition 2010, 26, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Bowry, V.W.; Frei, B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc. Natl. Acad. Sci. USA 1991, 88, 1646–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, S.J.; Chew, G.T.; Watts, G.F. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care 2009, 32, 810–812. [Google Scholar] [CrossRef] [Green Version]
- Watts, G.F.; Playford, D.A.; Croft, K.D.; Ward, N.C.; Mori, T.A.; Burke, V. Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia 2002, 45, 420–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiano, L.; Belardinelli, R.; Carnevali, P.; Principi, F.; Seddaiu, G.; Littarru, G.P. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: A double-blind, randomized controlled study. Eur. Heart J. 2007, 28, 2249–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raitakari, O.T.; McCredie, R.J.; Witting, P.; Griffiths, K.A.; Letters, J.; Sullivan, D.; Stocker, R.; Celermajer, D.S. Coenzyme Q improves LDL resistance to ex vivo oxidation but does not enhance endothelial function in hypercholesterolemic young adults. Free Radic. Biol. Med. 2000, 28, 1100–1105. [Google Scholar] [CrossRef]
- Langsjoen, P.H.; Langsjoen, A.M. Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin. Pharm. Drug Dev. 2014, 3, 13–17. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Williams, M.R.; Westerman, R.A.; Kingwell, B.A.; Paige, J.; Blombery, P.A.; Sudhir, K.; Komesaroff, P.A. Variations in endothelial function and arterial compliance during the menstrual cycle. J. Clin. Endocrinol. Metab. 2001, 86, 5389–5395. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Holvoet, P.; Vanhaecke, J.; Janssens, S.; Van de Werf, F.; Collen, D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998, 98, 1487–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Mao, Q.; Cao, J.; Wang, Y.; Zhou, X.; Fan, L. Effects of coenzyme Q10 on vascular endothelial function in humans: A meta-analysis of randomized controlled trials. Atherosclerosis 2012, 221, 311–316. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Bruno, R.M.; Bianchini, E.; Faita, F.; Taddei, S.; Ghiadoni, L. Intima media thickness, pulse wave velocity, and flow mediated dilation. Cardiovasc. Ultrasound 2014, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantino, S.; Paneni, F.; Battista, R.; Castello, L.; Capretti, G.; Chiandotto, S.; Tanese, L.; Russo, G.; Pitocco, D.; Lanza, G.A.; et al. Impact of Glycemic Variability on Chromatin Remodeling, Oxidative Stress, and Endothelial Dysfunction in Patients with Type 2 Diabetes and With Target HbA1c Levels. Diabetes 2017, 66, 2472–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; Lima, J.A.; Crouse, J.R.; Herrington, D.M. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation 2009, 120, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shechter, M.; Matetzky, S.; Arad, M.; Feinberg, M.S.; Freimark, D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur. J. Heart Fail. 2009, 11, 588–593. [Google Scholar] [CrossRef]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Iwamoto, A.; Kajikawa, M.; Matsumoto, T.; Oda, N.; et al. Brachial artery diameter as a marker for cardiovascular risk assessment: FMD-J study. Atherosclerosis 2018, 268, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Conraads, V.M.; Pattyn, N.; De Maeyer, C.; Beckers, P.J.; Coeckelberghs, E.; Cornelissen, V.A.; Denollet, J.; Frederix, G.; Goetschalckx, K.; Hoymans, V.Y.; et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: The SAINTEX-CAD study. Int. J. Cardiol. 2015, 179, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Early, K.S.; Stewart, A.; Johannsen, N.; Lavie, C.J.; Thomas, J.R.; Welsch, M. The Effects of Exercise Training on Brachial Artery Flow-Mediated Dilation: A Meta-analysis. J. Cardiopulm. Rehabil. Prev. 2017, 37, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Simental-Mendia, L.E.; Stefanutti, C.; Pirro, M. Supplementation with coenzyme Q10 reduces plasma lipoprotein(a) concentrations but not other lipid indices: A systematic review and meta-analysis. Pharm. Res. 2016, 105, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Georgiopoulos, G.; Chiriaco, M.; Grassi, G.; Seravalle, G.; Savoia, C.; Volpe, M.; Taddei, S.; Rizzoni, D.; Virdis, A. The importance of endothelial dysfunction in resistance artery remodelling and cardiovascular risk. Cardiovasc. Res. 2020, 116, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.; Farha, S.; Park, M.M.; Comhair, S.A.; Lundgrin, E.L.; Tang, W.H.; Bongard, R.D.; Merker, M.P.; Erzurum, S.C. Coenzyme Q supplementation in pulmonary arterial hypertension. Redox Biol. 2014, 2, 884–891. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Camacho, J.D.; Bernier, M.; Lopez-Lluch, G.; Navas, P. Coenzyme Q10 Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Belardinelli, R.; Mucaj, A.; Lacalaprice, F.; Solenghi, M.; Seddaiu, G.; Principi, F.; Tiano, L.; Littarru, G.P. Coenzyme Q10 and exercise training in chronic heart failure. Eur. Heart J. 2006, 27, 2675–2681. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, F.; Lazzarini, R.; Babini, L.; Prattichizzo, F.; Rippo, M.R.; Tiano, L.; Di Nuzzo, S.; Graciotti, L.; Festa, R.; Bruge, F.; et al. Anti-inflammatory effect of ubiquinol-10 on young and senescent endothelial cells via miR-146a modulation. Free Radic. Biol. Med. 2013, 63, 410–420. [Google Scholar] [CrossRef]
- Ghiadoni, L.; Faita, F.; Salvetti, M.; Cordiano, C.; Biggi, A.; Puato, M.; Di Monaco, A.; De Siati, L.; Volpe, M.; Ambrosio, G.; et al. Assessment of flow-mediated dilation reproducibility: A nationwide multicenter study. J. Hypertens. 2012, 30, 1399–1405. [Google Scholar] [CrossRef] [Green Version]



| Variable | Ubiquinol, 200 mg/day (n = 17) | Ubiquinol, 100 mg/day (n = 15) | Placebo (n = 16) |
|---|---|---|---|
| Age (years) | 58.2 (5.1) | 59.3 (6.4) | 59.6 (3.8) |
| Gender (males) | 7 | 5 | 9 |
| BMI (Kg/m2) | 23.8 (3.2) | 24.6 (3.6) | 24.1 (3.4) |
| Heart rate (bpm) | 64 (8) | 69 (7) | 67 (10) |
| Systolic blood pressure (mmHg) | 128 (15) | 129 (13) | 125 (12) |
| Diastolic blood pressure (mmHg) | 79 (7) | 82 (9) | 83 (10) |
| Hemoglobin (mg/dL) | 14.0 (1.0) | 14.6 (1.1) | 14.6 (1.0) |
| WBC (cells/mm3) | 5.97 (0.98) | 7.08 (0.98) | 6.67 (1.55) |
| Total cholesterol (mg/dL) | 214.5 (33.3) | 218.1 (29.4) | 229.8 (31.9) |
| LDL cholesterol (mg/dL) | 145.3 (25.7) | 147.0 (26.3) | 157.7 (29.4) |
| HDL cholesterol (mg/dL) | 59.3 (15.9) | 61.1 (14.8) | 62.6 (17.3) |
| Triglycerides (mg/dL) | 105.2 (59.1) | 109.7 (50.0) | 99.4 (70.4) |
| Glucose (mg/dL) | 95.2 (9.2) | 98.3 (9.3) | 96.7 (11.0) |
| HOMA index | 1.36 (0.65) | 1.12 (0.49) | 1.33 (0.57) |
| Creatinine (mg/dL) | 0.84 (0.15) | 0.89 (0.23) | 0.86 (0.16) |
| Alanine aminotransferase (U/L) | 21.0 (9.9) | 18.3 (9.9) | 18.6 (6.4) |
| Creatine kinase (U/L) | 108.3 (32.3) | 112.0 (45.1) | 365.5 (1009.5) |
| hs-CRP (mg/L) | 0.26 (0.27) | 0.11 (0.09) | 0.22 (0.27) |
| ESR (mm/h) | 15.1 (10.2) | 16.5 (10.1) | 10.8 (6.5) |
| Variable | Ubiquinol, 200 mg/day (n = 17) | Ubiquinol, 100 mg/day (n = 15) | Placebo (n = 16) |
|---|---|---|---|
| Baseline brachial artery diameter (mm) | 4.02 (1.0) | 3.97 (0.60) | 4.19 (1.02) |
| Flow-mediated dilation (%) | |||
| T0 (recruitment) | 3.48 (1.12) | 3.80 (0.95) | 4.06 (1.13) |
| T1 (week 4) | 4.34 (1.81) | 4.63 (0.90) | 4.51 (1.54) |
| T1-T0 difference | 0.86 (1.62) | 0.84 (1.16) | 0.45 (1.37) |
| T2 (week 8) | 4.75 (1.68) | 5.14 (1.12) | 3.65 (1.06) |
| T2-T0 difference ** | 1.28 (0.94) | 1.34 (1.44) | −0.41 (1.51) |
| T2-T1 difference * | 0.41 (1.48) | 0.51 (0.96) | −0,86 (1.52) |
| Effect size (Cohen’s d, 95% CI) | 0.89 (0.56–1.40) | 1.30 (0.38–2.09) | −0.37 (−1.10–0.20) |
| Variable | Ubiquinol, 200 mg/day (n = 17) | Ubiquinol, 100 mg/day (n = 15) | Placebo (n = 16) |
|---|---|---|---|
| Plasma CoQ10 (μmol/mol) | |||
| T0 (recruitment) | 108.7 (28.9) | 145.5 (80.3) | 131.1 (28.9) |
| T1 (week 4) | 712.0 (262.6) | 449.7 (310.1) | 120.7 (32.4) |
| T1-T0 difference *** | 603.3 (247.1) | 304.2 (256.6) | −10.4 (28.3) |
| T2 (week 8) | 723.0 (262.8) | 461.9 (245.9) | 121.2 (50.1) |
| T2-T0 difference *** | 614.4 (244.8) | 316.5 (202.5) | −9.9 (37.2) |
| T2-T1 difference | 11.1 (150.5) | 12.3 (180.6) | 0.5 (35.6) |
| Oxidized CoQ10 (%) | |||
| T0 (recruitment) | 12.6 (5.9) | 11.7 (4.7) | 11.6 (4.8) |
| T1 (week 4) | 6.0 (1.9) | 8.4 (4.2) | 11.1 (5.1) |
| T1-T0 difference *** | −6.6 (4.6) | −3.3 (3.8) | −0.5 (2.5) |
| T2 (week 8) | 7.2 (6.8) | 7.6 (2.4) | 14.1 (8.2) |
| T2-T0 difference *** | −5.4 (4.4) | −4.0 (3.1) | 2.5 (7.4) |
| T2-T1 difference | 1.2 (6.1) | −0.7 (2.8) | 3.0 (7.3) |
| Serum nitric oxide metabolites (μm) | |||
| T0 (recruitment) | 63.4 (16.6) | 63.7 (16.0) | 64.8 (15.3) |
| T2 (week 8) | 72.8 (20.0) | 69.5 (14.8) | 59.9 (15.6) |
| T2-T0 difference * | 9.3 (16.1) | 5.9 (11.9) | −4.9 (13.4) |
| Plasma oxidized LDL (U/L) | |||
| T0 (recruitment) | 74.0 (21.1) | 77.2 (16.4) | 85.2 (12.1) |
| T2 (week 8) | 82.6 (17.2) | 79.7 (17.2) | 80.2 (25.1) |
| T2-T0 difference | 8.6 (26.3) | 2.5 (23.5) | −5.0 (28.4) |
| LDL oxidation lag time (min) | |||
| T0 (recruitment) | 102.4 (30.2) | 104.3 (37.9) | 118.2 (31.9) |
| T2 (week 8) | 118.4 (43.2) | 100.7 (37.4) | 129.1 (40.1) |
| T2-T0 difference * | 16.0 (24.8) | −3.6 (24.3) | 11.0 (29.4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbatinelli, J.; Orlando, P.; Galeazzi, R.; Silvestri, S.; Cirilli, I.; Marcheggiani, F.; Dludla, P.V.; Giuliani, A.; Bonfigli, A.R.; Mazzanti, L.; et al. Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial. Nutrients 2020, 12, 1098. https://doi.org/10.3390/nu12041098
Sabbatinelli J, Orlando P, Galeazzi R, Silvestri S, Cirilli I, Marcheggiani F, Dludla PV, Giuliani A, Bonfigli AR, Mazzanti L, et al. Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial. Nutrients. 2020; 12(4):1098. https://doi.org/10.3390/nu12041098
Chicago/Turabian StyleSabbatinelli, Jacopo, Patrick Orlando, Roberta Galeazzi, Sonia Silvestri, Ilenia Cirilli, Fabio Marcheggiani, Phiwayinkosi V. Dludla, Angelica Giuliani, Anna Rita Bonfigli, Laura Mazzanti, and et al. 2020. "Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial" Nutrients 12, no. 4: 1098. https://doi.org/10.3390/nu12041098
APA StyleSabbatinelli, J., Orlando, P., Galeazzi, R., Silvestri, S., Cirilli, I., Marcheggiani, F., Dludla, P. V., Giuliani, A., Bonfigli, A. R., Mazzanti, L., Olivieri, F., Antonicelli, R., & Tiano, L. (2020). Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial. Nutrients, 12(4), 1098. https://doi.org/10.3390/nu12041098