Potential Nutrition and Health Claims in Deastringed Persimmon Fruits (Diospyros kaki L.), Variety ‘Rojo Brillante’, PDO ’Ribera del Xúquer’
Abstract
:1. Introduction
2. Materials and Methods
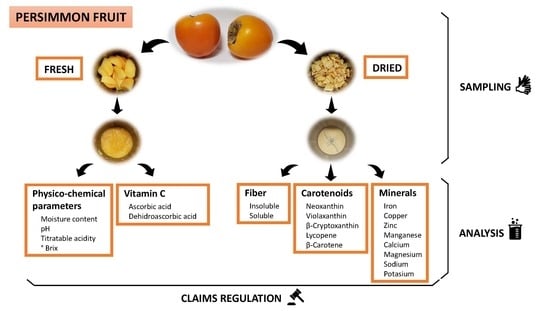
2.1. Sampling
2.2. Analytical Methods
2.2.1. Fiber Analysis
2.2.2. Vitamin C Analysis
2.2.3. Carotenoids Analysis
Ultrasound-Assisted Extraction (UAE)
Chromatographic Conditions
Standard Carotenoid Preparation
2.2.4. Mineral Composition Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameters
3.2. Fiber Content
3.3. Vitamin C Content
3.4. Carotenoid Profile
3.5. Mineral Composition
3.6. Potential Nutrition Claims Applied to Deastringed ‘Rojo Brillante’ Persimmon Fruits (Diospyros kaki L.), Protected Designation of Origin (PDO) ‘Ribera del Xúquer’
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arroyo, U.P.; Mazquiaran, B.L.; Rodríguez, A.P.; Valero, G.T.; Ruiz, M.E.; Ávila, T.J.M.; Varela-Moreiras, G. Informe de estado de situación sobre frutas y hortalizas: Nutrición y salud en la España del siglo XXI; Fundación Española de la Nutrición: Madrid, Spain, 2018; Available online: http://www.fen.org.es/storage/app/media/imgPublicaciones/INFORME_FRUTAS_Y_HORTALIZAS_FEN_2018.pdf (accessed on 14 August 2018).
- Kluge, R.; Tessmer, M. Caqui, Diospyros kaki. In Exotic Fruits, 1st ed.; Rodrigues, S., Silva, E., de Brito, E., Eds.; Academic Press: Massachusetts, UK, 2018; pp. 113–119. [Google Scholar]
- Persimmon. Available online: https://www.tridge.com/intelligences/persimmon (accessed on 3 January 2020).
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef]
- Conoce la D.O.P. -Kaki Persimon®. Available online: http://persimon.eu/dop-ribera-del-xuquer/conoce-la-dop/ (accessed on 2 January 2020).
- European Parliament and Council of the European Union. Regulation (EU) No 1151/2012 of the European Parliament and of the Council of 21 November 2012 on quality schemes for agricultural products and foodstuffs. Off. J. Eur. Union 2012, 343, 1–29. [Google Scholar]
- Arnal, L.; Besada, C.; Navarro, P.; Salvador, A. Effect of controlled atmospheres on maintaining quality of persimmon fruit cv. “Rojo Brillante”. J. Food Sci. 2008, 73, S26–S30. [Google Scholar] [CrossRef]
- Novillo, P.; Besada, C.; Tian, L.; Bermejo, A.; Salvador, A. Nutritional composition of ten persimmon cultivars in the “ready-to-eat crisp” stage. Effect of deastringency treatment. FNS 2015, 6, 1296–1306. [Google Scholar] [CrossRef] [Green Version]
- Salvador, A.; Arnal, L.; Besada, C.; Larrea, V.; Quiles, A.; Pérez-Munuera, I. Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv. ‘Rojo Brillante’. Postharvest Biol. Technol. 2007, 46, 181–188. [Google Scholar] [CrossRef]
- Munera, S.; Aleixos, N.; Besada, C.; Gómez-Sanchis, J.; Salvador, A.; Cubero, S.; Talens, P.; Blasco, J. Discrimination of astringent and deastringed hard ‘Rojo Brillante’ persimmon fruit using a sensory threshold by means of hyperspectral imaging. J. Food Eng. 2019, 263, 173–180. [Google Scholar] [CrossRef]
- Plaza, L.; Colina, C.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.). Food Chem. 2012, 130, 591–597. [Google Scholar] [CrossRef]
- Yamasaki, A.; Yakushiji, H.; Iwamoto, H.; Azuma, A.; Sugiura, H. Optimum Conditions for On-tree Ethanol-sticker Treatment for Astringency Removal in ‘Taigetsu’ and ‘Taiten’ Persimmons. Hortic. J. 2017, 86, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Effect of particle size on phytochemical composition and antioxidant properties of two persimmon flours from Diospyros kaki Thunb. Vars. ‘Rojo Brillante’ and ‘Triumph’co-products. J. Sci. 2018, 98, 504–510. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council of the European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and heath claims made on food. Off. J. Eur. Union 2006, 404, 9–25. [Google Scholar]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. The frontier between nutrition and pharma: The international regulatory framework of functional foods, food supplements and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2019, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Off. J. Eur. Union 2011, 304, 18–63. [Google Scholar]
- Latimer, G.W. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Association of Analytical Communities (AOAC). Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Horwitz, W.; Latimer, G.W.; Association of Official Analytical Chemists International. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Divakaran, D.; Chandran, A.; Chandran, R.P. Comparative study on production of α-Amylase from Bacillus licheniformis strains. Braz. J. Microbiol. 2011, 42, 1397–1404. [Google Scholar] [CrossRef] [Green Version]
- García Herrera, P.; Sánchez-Mata, M.C.; Cámara, M. Nutritional Characterization of tomato fiber as a useful ingredient for food industry. Innov. Food Sci. Emerg. 2010, 11, 707–711. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Cámara-Hurtado, M.; Diez-Marques, C.; Torija-Isasa, M.E. Comparison of HPLC and spectrofluorimetry for vitamin C analysis of green beans. Eur. Food Res. Technol. 2000, 210, 220–225. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Cabrera Loera, R.D.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Díez Marqués, C.; Pardo-de-Santayana, M.; Tardío, J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop. Evolut. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- Olives Barba, A.I.; Cámara Hurtado, M.; Sánchez Mata, M.C.; Fernández Ruiz, V.; López Sáenz de Tejada, M. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Mahan, L.K.; Escott-Stump, S.; Raymond, J.L. Krause Dietoterapia, 13th ed.; Elsevier: Madrid, Spain, 2012. [Google Scholar]
- Spanish Food Composition Database (BEDCA). Available online: http://www.bedca.net (accessed on 4 January 2020).
- FRIDA Database. Available online: https://frida.fooddata.dk/ (accessed on 4 January 2020).
- Janick, J.; Paull, R.E. The Encyclopedia of Fruit & Nuts, 1st ed.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Mataix, J.; Mañas, M.; Llopis, J.; Martínez, E. Tabla de composición de alimentos españoles. Servicio de, 2nd ed.; Publicaciones de la Universidad de Granada: Campus Universitario de Cartuja, Granada, Spain, 1995. [Google Scholar]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de composición de alimentos. Guía de Prácticas, 9th ed.; Ediciones Pirámide: Madrid, Spain, 2018. [Google Scholar]
- Rizza, R.; Liang, V.; Molly, M.; Harrison, G. Encyclopedia of Foods: A Guide to Healthy Nutrition, 1st ed.; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Souci, S.; Fachmann, W.; Kraut, H.; Kirchhoff, E. Food Composition and Nutrition Tables, 17th ed.; MedPharm Scientific Publishers: Stuttgart, Germany, 2008. [Google Scholar]
- USDA—Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/foods (accessed on 4 January 2020).
- Cámara, M.; Fernández-Ruiz, V.; Morales, P.; Sánchez-Mata, M.C. Fibre Compounds and Human Health. Curr. Pharm. Des. 2017, 23, 2835–2849. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara Hurtado, M. Phytochemicals and Nutraceuticals with Particular Health-Promoting Effects. Pharmatech 2020, 48, 74–78. [Google Scholar]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R. Dietary fiber in foods: A review. JFST 2011, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority (EFSA). Dietary Reference Values for Nutrients Summary Report; EFSA Supporting Publications: Parma, Italy, 2017; p. e15121E. [Google Scholar] [CrossRef] [Green Version]
- Cámara, M.; Sánchez-Mata, M.C.; Torija, M.E. Frutas y verduras fuentes de salud. Colección Nutrición y Salud. Servicio de Promoción de la Salud; Instituto de Salud Pública. Consejería de Sanidad y Consumo Comunidad de Madrid: Madrid, Spain, 2003; Volume 8, p. 73. ISBN 84-688-4713-5. [Google Scholar]
- Mahan, L.K.; Escott-Stump, S.; González Hernández, J.L. Nutrición y dietoterapia de, Krause, 10th ed.; McGraw-Hill Interamericana: Mexico D.F., Mexico, 2001; ISBN 9701032047. [Google Scholar]
- Novillo, P.; Salvador, A.; Magalhaes, T.; Besada, C. Deastringency treatment with CO2 induces oxidative stress in persimmon fruit. Postharvest Biol. Technol. 2014, 92, 16–22. [Google Scholar] [CrossRef]
- Prakash, D.; Gupta, C. Phytochemicals of Nutraceutical Importance. In Carotenoids: Chemistry and Health Benefits, 1st ed.; CAB International: Wallingford, UK, 2014. [Google Scholar]
- Jaswir, I.; Noviendri, D.; Fitri, R.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plant. Res. 2011, 5, 7119–7131. [Google Scholar] [CrossRef]
- Cámara, M.; Sánchez-Mata, M.C.; Fernández-Ruiz, V.; Cámara, R.M.; Manzoor, S.; Cáceres, J.O. Lycopene. In A Review of Chemical and Biological Activity Related to Beneficial Health Effects, 1st ed.; Elsevier: Oxford, UK, 2013; pp. 383–426. [Google Scholar]
- Giordani, E.; Doumett, S.; Nin, S.; Del Bubba, M. Selected primary and secondary metabolites in fresh persimmon (Diospyros kaki Thunb.): A review of analytical methods and current knowledge of fruit composition and health benefits. Food Res. Int. 2011, 44, 1752–1767. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Oliveras, M.J.; Quesada, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res. Int. 2018, 105, 461–472. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, D.; Sheng, Y.; Tao, J.; Yang, Y. Carotenoids in fruits of different persimmon cultivars. Molecules 2011, 16, 624–636. [Google Scholar] [CrossRef] [Green Version]
- Dinicolantonio, J.; Liu, J.; O’Keefe, J. Magnesium for the prevention and treatment of cardiovascular disease. Open Heart 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Drago, S.R. Minerals. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques, 1st ed.; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 129–157. [Google Scholar]
- Kohlmeier, M. Minerals and Trace Elements. In Nutrient Metabolism: Structures, Functions, and Genes, 1st ed.; Kohlmeier, M., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 673–807. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 136, 1. [Google Scholar]

| Compound | Solvent Stock Solutions | Solvent Work Solution | RT (min) | Range of Linearity (µg/mL) | Correlation Coefficient (R2) | LD | LQ |
|---|---|---|---|---|---|---|---|
| Neoxanthin | Ethyl eter | ACN | 5.57 | 2.5–20 | 0.9915 | 2.60 | 8.69 |
| Violaxanthin | Ethyl eter | ACN | 6.15 | 0.1–5 | 0.9955 | 0.51 | 1.70 |
| β-Cryptoxanthin | Cloroformo | Hexane | 15.69 | 1–15 | 0.9992 | 0.15 | 0.52 |
| Lycopene | THF:ACN:MetOH (15:30:55, v:v:v) | Hexane | 19.14 | 2–20 | 0.9969 | 0.83 | 2.76 |
| β-Carotene | Cloroformo | Hexane | 28.57 | 0.25–5 | 0.9984 | 0.15 | 0.509 |
| Batch | Moisture (g/100 g) | pH | Acidity (meq NaOH/100 g) | °Brix |
|---|---|---|---|---|
| 2017 season | ||||
| K1 | 81.04 ± 0.08 | 6.34 ± 0.02 | 0.40 ± 0.00 | 16.00 ± 0.10 |
| K2 | 81.47 ± 0.10 | 6.27 ± 0.04 | 1.11 ± 0.01 | 19.37 ± 0.12 |
| K3 | 82.01 ± 0.40 | 5.84 ± 0.05 | 0.78 ± 0.03 | 18.73 ± 0.12 |
| 2018 season | ||||
| K4 | 81.84 ± 0.02 | 6.29 ± 0.04 | 2.87 ± 0.17 | 17.10 ± 0.10 |
| K5 | 83.07 ± 0.05 | 6.24 ± 0.08 | 2.94 ± 0.17 | 15.77 ± 0.06 |
| K6 | 82.23 ± 0.07 | 6.07 ± 0.07 | 3.23 ± 0.29 | 16.57 ± 0.12 |
| Range | 81.04–83.07 | 5.84–6.34 | 0.40–3.23 | 15.77–19.37 |
| Batch | Insoluble Fiber (mg/100 g) | Soluble Fiber (mg/100 g) | Total Dietary Fiber (mg/100 g) | Total Dietary Fiber (mg/100 kcal) |
|---|---|---|---|---|
| 2017 season | ||||
| K1 | 1.71 ± 0.13 | 3.20 ± 0.19 | 4.90 ± 0.34 | 6.57 ± 0.46 |
| K2 | 2.79 ± 0.26 | 2.20 ± 0.15 | 4.99 ± 0.26 | 6.69 ± 0.35 |
| K3 | 2.60 ± 0.17 | 2.31 ± 0.17 | 4.47 ± 0.26 | 5.99 ± 0.26 |
| 2018 season | ||||
| K4 | 1.17 ± 0.02 | 1.14 ± 0.06 | 2.38 ± 0.14 | 3.19 ± 0.19 |
| K5 | 0.89 ± 0.03 | 1.64 ± 0.12 | 2.54 ± 0.10 | 3.41 ± 0.13 |
| K6 | 0.86 ± 0.02 | 1.85 ± 0.18 | 2.72 ± 0.19 | 3.65 ± 0.25 |
| Range | 0.86–2.79 | 1.14–3.20 | 2.38–4.99 | 3.19–6.69 |
| Batch | Ascorbic Acid (AA) (mg/100 g) | Dehydroascorbic Acid (DHA) (mg/100 g) | Total Vitamin C (mg/100 g) |
|---|---|---|---|
| K1 | 1.56 ± 0.49 | 7.71 ± 0.60 | 9.43 ± 1.04 |
| K2 | 1.45 ± 0.01 | 8.80 ± 0.76 | 10.25 ± 0.76 |
| K3 | 1.22 ± 0.19 | 3.56 ± 0.70 | 4.62 ± 0.41 |
| Range | 1.22–1.56 | 3.56–8.80 | 4.62–10.25 |
| Batch | Neoxanthin (µg/100 g) | Violaxanthin (µg/100 g) | β-Cryptoxanthin (µg/100 g) | Lycopene (µg/100 g) | β-Carotene (µg/100 g) | Total Carotenoids (µg/100 g) | RE (µg/100 g) |
|---|---|---|---|---|---|---|---|
| 2017 season | |||||||
| K1 | 3.18 ± 0.25 | 0.06 ± 0.02 | 1.76 ± 0.41 | 26.60 ± 3.40 | 10.60 ± 0.70 | 40.86 ± 4.31 | 3.84 ± 0.12 |
| K2 | 4.23 ± 1.92 | 0.09 ± 0.01 | 3.07 ± 0.55 | 53.50 ± 4.11 | 20.50 ± 3.86 | 75.84 ± 5.33 | 4.21 ± 0.06 |
| K3 | 1.08 ± 0.34 | 0.04 ± 0.04 | 2.77 ± 0.53 | 42.20 ± 1.31 | 15.55 ± 0.62 | 60.24 ± 1.54 | 3.97 ± 0.00 |
| 2018 season | |||||||
| K4 | nd | 0.03 ± 0.01 | 1.75 ± 0.62 | 17.51 ± 6.71 | 12.38 ± 3.44 | 47.46 ± 10.12 | 1.92 ± 0.07 |
| K5 | nd | nd | 0.75 ± 0.41 | 26.74 ± 3.43 | 10.07 ± 2.54 | 35.48 ± 6.37 | 1.61 ± 0.08 |
| K6 | nd | nd | 1.32 ± 0.40 | 41.97 ± 5.12 | 13.26 ± 5.12 | 41.38 ± 10.61 | 1.66 ± 0.13 |
| Range | nd–4.23 | nd–0.09 | 0.75–3.07 | 17.51–53.50 | 10.07–20.50 | 35.48–75.84 | 1.61–4.21 |
| Batch | Fe (mg/100 g) | Cu (mg/100 g) | Zn (mg/100 g) | Mn (mg/100 g) | Ca (mg/100 g) | Mg (mg/100 g) | Na (mg/100 g) | K (mg/100 g) |
|---|---|---|---|---|---|---|---|---|
| 2017 season | ||||||||
| K1 | 0.15 ± 0.00 | 0.26 ± 0.01 | 0.11 ± 0.01 | 0.07 ± 0.01 | 14.41 ± 0.48 | 13.65 ± 0.38 | 3.84 ± 0.12 | 251.24 ± 3.20 |
| K2 | 0.19 ± 0.02 | 0.28 ± 0.03 | 0.26 ± 0.01 | 0.06 ± 0.01 | 14.17 ± 0.51 | 19.50 ± 0.96 | 4.21 ± 0.06 | 279.67 ± 24.43 |
| K3 | 0.23 ± 0.00 | 0.28 ± 0.00 | 0.69 ± 0.07 | 0.06 ± 0.01 | 36.63 ± 2.39 | 20.95 ± 1.49 | 3.97 ± 0.00 | 349.11 ± 1.46 |
| 2018 season | ||||||||
| K4 | 4.12 ± 0.33 | 3.93 ± 0.17 | 0.35 ± 0.03 | 0.25 ± 0.00 | 12.56 ± 0.21 | 7.78 ± 0.76 | 1.92 ± 0.07 | 101.30 ± 10.10 |
| K5 | 4.13 ± 0.33 | 3.36 ± 0.02 | 0.35 ± 0.03 | 0.23 ± 0.02 | 10.36 ± 0.33 | 7.03 ± 0.12 | 1.61 ± 0.08 | 104.00 ± 8.76 |
| K6 | 4.17 ± 0.39 | 3.33 ± 0.29 | 0.30 ± 0.03 | 0.26 ± 0.02 | 11.09 ± 1.04 | 7.78 ± 0.55 | 1.66 ± 0.13 | 104.36 ± 10.40 |
| Range | 0.15–4.17 | 0.26–3.93 | 0.11–0.69 | 0.06–0.26 | 10.36–36.63 | 7.03–20.95 | 1.61–4.21 | 101.30–349.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez Díaz, L.; Dorta, E.; Maher, S.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Sánchez-Mata, M.-C. Potential Nutrition and Health Claims in Deastringed Persimmon Fruits (Diospyros kaki L.), Variety ‘Rojo Brillante’, PDO ’Ribera del Xúquer’. Nutrients 2020, 12, 1397. https://doi.org/10.3390/nu12051397
Domínguez Díaz L, Dorta E, Maher S, Morales P, Fernández-Ruiz V, Cámara M, Sánchez-Mata M-C. Potential Nutrition and Health Claims in Deastringed Persimmon Fruits (Diospyros kaki L.), Variety ‘Rojo Brillante’, PDO ’Ribera del Xúquer’. Nutrients. 2020; 12(5):1397. https://doi.org/10.3390/nu12051397
Chicago/Turabian StyleDomínguez Díaz, Laura, Eva Dorta, Sarita Maher, Patricia Morales, Virginia Fernández-Ruiz, Montaña Cámara, and María-Cortes Sánchez-Mata. 2020. "Potential Nutrition and Health Claims in Deastringed Persimmon Fruits (Diospyros kaki L.), Variety ‘Rojo Brillante’, PDO ’Ribera del Xúquer’" Nutrients 12, no. 5: 1397. https://doi.org/10.3390/nu12051397
APA StyleDomínguez Díaz, L., Dorta, E., Maher, S., Morales, P., Fernández-Ruiz, V., Cámara, M., & Sánchez-Mata, M.-C. (2020). Potential Nutrition and Health Claims in Deastringed Persimmon Fruits (Diospyros kaki L.), Variety ‘Rojo Brillante’, PDO ’Ribera del Xúquer’. Nutrients, 12(5), 1397. https://doi.org/10.3390/nu12051397