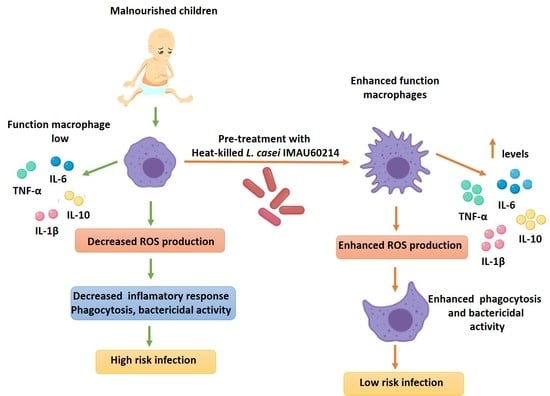
Impact of Heat-Killed Lactobacillus casei Strain IMAU60214 on the Immune Function of Macrophages in Malnourished Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects and Biological Sample Collection
2.2. Culture of Monocyte-Derived Macrophage (MDM)
2.3. Bacterial Strain
2.4. Pre-Treatment of MDM Cells with Heat-Killed L. casei IMAU60214
2.5. Chemiluminescence
2.6. Cytokine Secretion
2.7. Examination of the Phagocyte Function of MDM Cells
2.8. Activity Bactericidal Assay
2.9. Statistical Analysis
3. Results
3.1. Effect of Heat-Killed L. casei IMAU60214 on the Secretion of ROS on MDM Cells from WNH, WNI and MNI Chlidren
3.2. Heat-Killed L. casei IMAU60214 Induced Levels of Cytokine Secretion in Vitro on MDM Cells from WNH, WNI and MNI Chlidren
3.3. Heat-Killed L. casei IMAU60214 Increase the Phagocytic Function In Vitro of MDM Cells from WNH, WNI and MNI Chlidren
3.4. Heat-Killed L. casei IMAU60214 Increases the In Vitro Bactericidal Activity of MDM Cells from WNH, WNI and MNI Chlidren
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chinen, J.; Shearer, W.T. Secondary immunodeficiencies, including HIV infection. J. Allergy Clin. Immunol. 2010, 125, S195–S203. [Google Scholar] [CrossRef]
- United Nations Administrative Committee on Coordination. Fourth Report on the World Nutrition Situation; United Nations Administrative Committee on Coordination/Sub-Committee on Nutrition: Geneva, Switzerland, 2000. [Google Scholar]
- Uwaezuoke, S.N.; Ndu, I.K.; Eze, I.C. The prevalence and risk of urinary tract infection in malnourished children: A systematic review and meta-analysis. BMC Pediatr. 2019, 19, 261. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.C.; German, E.; Ferreira, D.M.; Rylance, J. Nasopharyngeal colonization with streptococcus pneumoniae in malnourished children: A systematic review and meta-analysis of prevalence. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 227–233. [Google Scholar] [CrossRef]
- Olofin, I.; McDonald, C.M.; Ezzati, M.; Flaxman, S.; Black, R.E.; Fawzi, W.W.; Caulfield, L.E.; Danaei, G.; Nutrition Impact Model Study (anthropometry cohort pooling). Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: A pooled analysis of ten prospective studies. PLoS ONE 2013, 8, e64636. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of childhood malnutrition on host defense and infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef] [Green Version]
- Latham, M.C. Protein-Energy malnutrition—Its epidemiology and control. J. Environ. Pathol Toxicol. Oncol. 1990, 10, 168–180. [Google Scholar]
- Jones, K.D.; Berkley, J.A. Severe acute malnutrition and infection. Paediatr. Int. Child Health 2014, 34, S1–S29. [Google Scholar] [CrossRef]
- Bourke, C.D.; Jones, K.D.J.; Prendergast, A.J. Current understanding of innate immune cell dysfunction in childhood undernutrition. Front. Immunol. 2019, 10, 1728. [Google Scholar] [CrossRef] [Green Version]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, A.J. Malnutrition and vaccination in developing countries. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140141. [Google Scholar] [CrossRef] [Green Version]
- Savy, M.; Edmond, K.; Fine, P.E.; Hall, A.; Hennig, B.J.; Moore, S.E.; Mulholland, K.; Schaible, U.; Prentice, A.M. Landscape analysis of interactions between nutrition and vaccine responses in children. J. Nutr. 2009, 139, 2154s–2218s. [Google Scholar] [CrossRef] [Green Version]
- Fock, R.A.; Vinolo, M.A.; Crisma, A.R.; Nakajima, K.; Rogero, M.M.; Borelli, P. Protein-Energy malnutrition modifies the production of interleukin-10 in response to lipopolysaccharide (LPS) in a murine model. J. Nutr. Sci. Vitaminol. (Tokyo) 2008, 54, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Redmond, H.P.; Leon, P.; Lieberman, M.D.; Hofmann, K.; Shou, J.; Reynolds, J.V.; Goldfine, J.; Johnston, R.B.; Daly, J.M. Impaired macrophage function in severe protein-energy malnutrition. Arch. Surg. 1991, 126, 192–196. [Google Scholar] [CrossRef]
- Rodríguez, L.; González, C.; Flores, L.; Jiménez-Zamudio, L.; Graniel, J.; Ortiz, R. Assessment by flow cytometry of cytokine production in malnourished children. Clin. Diagn. Lab. Immunol. 2005, 12, 502–507. [Google Scholar] [CrossRef] [Green Version]
- González-Torres, C.; González-Martínez, H.; Miliar, A.; Nájera, O.; Graniel, J.; Firo, V.; Alvarez, C.; Bonilla, E.; Rodríguez, L. Effect of malnutrition on the expression of cytokines involved in Th1 cell differentiation. Nutrients 2013, 5, 579–593. [Google Scholar] [CrossRef] [Green Version]
- Lesourd, B.; Mazari, L. Nutrition and immunity in the elderly. Proc. Nutr. Soc. 1999, 58, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Morais, N.G.; Costa, T.B.D.; Ferreira de Lima, L.F.; dos Santos Basílio, D.; de Morais, N.N.G.; de Paiva Cavalcanti, M.; Pereira, V.R.A.; de Castro, C.M.M.B. Impact of neonatal malnutrition on expression TLR-9, NF-kB and cytokines of macrophages infected in vitro with methicillin resistant Staphylococcus aureus. Microb. Pathog. 2019, 132, 254–260. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; Bacarea, A.; Marusteri, M.; Bacarea, V.; Constantin, M.; Manolache, M. Efficacy and safety of APT198K for the treatment of infantile colic: A pilot study. J. Comp. Eff. Res. 2017, 6, 137–144. [Google Scholar] [CrossRef]
- Burta, O.; Iacobescu, C.; Mateescu, R.B.; Nicolaie, T.; Tiuca, N.; Pop, C.S. Efficacy and safety of APT036 versus simethicone in the treatment of functional bloating: A multicentre, randomised, double-blind, parallel group, clinical study. Transl. Gastroenterol. Hepatol. 2018, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Athalye-Jape, G.; Patole, S. Para-Probiotics for preterm neonates—The next frontier. Nutrients 2018, 10, 871. [Google Scholar] [CrossRef] [Green Version]
- Kataria, J.; Li, N.; Wynn, J.L.; Neu, J. Probiotic microbes: Do they need to be alive to be beneficial? Nutr. Rev. 2009, 67, 546–550. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef] [Green Version]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Mandal, S. Bifidobacteria-Insight into clinical outcomes and mechanisms of its probiotic action. Microbiol. Res. 2016, 192, 159–171. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from bifidobacterium and lactobacilluswithin the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, K.; Kawase, M.; Kubota, A.; Yoda, K.; Harata, G.; Hosoda, M.; He, F. Heat-Killed Lactobacillus gasseri can enhance immunity in the elderly in a double-blind, placebo-controlled clinical study. Benef. Microbes 2015, 6, 441–449. [Google Scholar] [CrossRef]
- Hatcher, G.E.; Lambrecht, R.S. Augmentation of macrophage phagocytic activity by cell-free extracts of selected lactic acid-producing bacteria. J. Dairy Sci. 1993, 76, 2485–2492. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tsen, H.Y.; Lin, C.L.; Lin, C.K.; Chuang, L.T.; Chen, C.S.; Chiang, Y.C. Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. J. Med. Microbiol. 2013, 62, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Habil, N.; Abate, W.; Beal, J.; Foey, A.D. Heat-Killed probiotic bacteria differentially regulate colonic epithelial cell production of human β-defensin-2: Dependence on inflammatory cytokines. Benef. Microbes 2014, 5, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kim, J.H.; Yang, H.; Dal Kang, S.; Song, S.; Lee, D.; Lee, J.S.; Park, J.H.Y.; Byun, S.; Lee, K.W. Heat-Killed Lactobacillus plantarum KCTC 13314BP enhances phagocytic activity and immunomodulatory effects via activation of MAPK and STAT3 pathways. J. Microbiol. Biotechnol. 2019, 29, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Galvan, R. Pediatric somatometry. Semilongituginal study of children in Mexico City. Arch. Investig. Med. (Mex) 1975, 6, 83–396. [Google Scholar] [PubMed]
- World Health Organization. Community-Based Management of Severe Acute Malnutrition; World Food Programme, United Nations System Standing Committee on Nutrition & United Nations Children’s Fund WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Rellstab, P.; Schaffner, A. Endotoxin suppresses the generation of O2- and H2O2 by “resting” and lymphokine-activated human blood-derived macrophages. J. Immunol. 1989, 142, 2813–2820. [Google Scholar]
- 38. Cruz-Guerrero, A.; Hernández-Sánchez, H.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; García-Garibay, M.; Figueroa-González, I. Commercial probiotic bacteria and prebiotic carbohydrates: A fundamental study on prebiotics uptake, antimicrobials production and inhibition of pathogens. J. Sci. Food Agric. 2014, 94, 2246–2252. [Google Scholar] [CrossRef]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Moreno Guerrero, S.S.; Ramírez Pacheco, A.; García Garibay, M.; Eslava, C. Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef] [Green Version]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.J. Changes in nutritional status impact immune cell metabolism and function. Front. Immunol. 2018, 9, 1055. [Google Scholar] [CrossRef] [Green Version]
- Soldan, S.P.; Zalles, C.L.; Eróstegui, R.C.; Olguín, A.; Sevilla, E.G. Efecto del Lactobacillus rhamnosus GG en la recuperación inmunonutricional de niños desnutridos graves. Gac. Médica Boliv. 2011, 34, 71–75. [Google Scholar]
- Douglas, S.D.; Schopfer, K. Phagocyte function in protein-calorie malnutrition. Clin. Exp. Immunol. 1974, 17, 121–128. [Google Scholar]
- Carvalho Neves, F.W.; Martins Campos, J.V.; Carneiro, L.R. Non specific immunological response in moderate malnutrition. Allergol. Immunopathol. (Madr) 1984, 12, 489–496. [Google Scholar]
- Keusch, G.T.; Douglas, S.D.; Hammer, G.; Braden, K. Antibacterial functions of macrophages in experimental protein-calorie malnutrition. II. Cellular and humoral factors for chemotaxis, phagocytosis, and intracellular bactericidal activity. J. Infect. Dis. 1978, 138, 134–142. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.B.D.; Morais, N.G.; Pedrosa, A.L.F.; Suênia Da Cunha, G.; De Castro, M.C.A.; Pereira, V.R.A.; Cavalcanti, M.D.P.; De Castro, C.M.M. Neonatal malnutrition programs the oxidant function of macrophages in response to Candida albicans. Microb. Pathog. 2016, 95, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Tomusiak-Plebanek, A.; Heczko, P.; Skowron, B.; Baranowska, A.; Okoń, K.; Thor, P.J.; Strus, M. Lactobacilli with superoxide dismutase-like or catalase activity are more effective in alleviating inflammation in an inflammatory bowel disease mouse model. Drug Des. Dev. Ther. 2018, 12, 3221–3233. [Google Scholar] [CrossRef] [Green Version]
- Noureen, S.; Riaz, A.; Arshad, M.; Arshad, N. In vitro selection and in vivo confirmation of the antioxidant ability of Lactobacillus brevis MG000874. J. Appl. Microbiol. 2019, 126, 1221–1232. [Google Scholar] [CrossRef]
- Cheng, X.L.; Ding, F.; Li, H.; Tan, X.Q.; Liu, X.; Cao, J.M.; Gao, X. Activation of AMPA receptor promotes TNF-α release via the ROS-cSrc-NFκB signaling cascade in RAW264.7 macrophages. Biochem. Biophys. Res. Commun. 2015, 461, 275–280. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Dostert, C.; Ludigs, K.; Guarda, G. Innate and adaptive effects of inflammasomes on T cell responses. Curr. Opin. Immunol. 2013, 25, 359–365. [Google Scholar] [CrossRef]
- Kaji, R.; Kiyoshima-Shibata, J.; Tsujibe, S.; Nanno, M.; Shida, K. Short communication: Probiotic induction of interleukin-10 and interleukin-12 production by macrophages is modulated by co-stimulation with microbial components. J. Dairy Sci. 2018, 101, 2838–2841. [Google Scholar] [CrossRef] [PubMed]
- Stunault, M.I.; Bories, G.; Guinamard, R.R.; Ivanov, S. Metabolism plays a key role during macrophage activation. Mediat. Inflamm. 2018, 2426138. [Google Scholar] [CrossRef] [PubMed]
- Kolling, Y.; Salva, S.; Villena, J.; Alvarez, S. Are the immunomodulatory properties of Lactobacillus rhamnosus CRL1505 peptidoglycan common for all Lactobacilli during respiratory infection in malnourished mice? PLoS ONE 2018, 13, e0194034. [Google Scholar] [CrossRef] [PubMed]
- Kolling, Y.; Salva, S.; Villena, J.; Marranzino, G.; Alvarez, S. Non-Viable immunobiotic Lactobacillus rhamnosus CRL1505 and its peptidoglycan improve systemic and respiratory innate immune response during recovery of immunocompromised-malnourished mice. Int. Immunopharmacol. 2015, 25, 474–484. [Google Scholar] [CrossRef]
- Villena, J.; Barbieri, N.; Salva, S.; Herrera, M.; Alvarez, S. Enhanced immune response to pneumococcal infection in malnourished mice nasally treated with heat-killed Lactobacillus casei. Microbiol Immunol. 2009, 53, 636–646. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Jiang, H.; Wei, M.; Liang, S.; Wang, M.; Shi, K.; He, Q. Macrophages are involved in gut bacterial translocation and reversed by Lactobacillus in experimental uremia. Dig. Dis. Sci. 2016, 61, 1534–1544. [Google Scholar] [CrossRef]
- Kaushal, D.; Kansal, V.K. Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum improves phagocytic potential of macrophages in aged mice. J. Food Sci. Technol. 2014, 51, 1147–1153. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Singh, T.P.; Malik, R.K. In vivo implications of potential probiotic Lactobacillus reuteri LR6 on the gut and immunological parameters as an adjuvant against protein energy malnutrition. Probiotics Antimicrob. Proteins 2019, 12, 517–534. [Google Scholar] [CrossRef]
- Campeotto, F.; Suau, A.; Kapel, N.; Magne, F.; Viallon, V.; Ferraris, L.; Waligora-Dupriet, A.J.; Soulaines, P.; Leroux, B.; Kalach, N.; et al. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 2011, 105, 1843–1851. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.A.; Kim, H.; Lee, K.W.; Park, K.Y. Dead lactobacillus plantarum stimulates and skews immune responses toward T helper 1 and 17 polarizations in RAW 264.7 cells and MOUSE SPLENOCYTES. J. Microbiol. Biotechnol. 2016, 26, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, I.; Choi, J.W.; Lee, C.W.; Cho, S.; Choi, T.G.; Sohn, M.; Park, Y.I. Micronized and heat-treated Lactobacillus plantarum LM1004 stimulates host immune responses via the TLR-2/MAPK/NF-κB signalling pathway in vitro and in vivo. J. Microbiol. Biotechnol. 2019, 29, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Malagón-Rojas, J.N.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for preventing and treating common infectious diseases in children: A systematic review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Characteristics Cohort | Well-Nourished Healthy Children | Well-Nourished Infected Children | Malnourished Infected Children |
|---|---|---|---|
| Patients (N) | 15 | 15 | 15 |
| Girls/Boys, n | 7(46%)/8(53%) | 6(40%)/9(60%) | 3(20%)/12(80%) |
| Age (months) | 16.4 (±6.4) | 15.7 (±7.5) | 14.4 (±6.1) |
| Weight (kg) | 12.5 (±0.5) | 11 (±0.3) | 5.4 (±0.2) |
| Height (cm) | 83 (±1.2)/92.5 (±3) | 82 (±3.4)/87.5 (±2.5) | 75 (±2)/80 (±3.4) |
| Z-score (WHZ) | Z > −1 to Z < 1 | Z > 0 to Z < 1 | Z < −3 |
| Clinical malnutrition a | Normal | Normal | Severe malnutrition |
| (Non-edematous marasmus) | - | - | 15 |
| Respiratory tract infection | - | 12 | 11 |
| Multiple abscesses | - | 1 | - |
| Diarrhea | - | - | 3 |
| Periorbital cellulitis | - | 1 | - |
| Bacterial meningitis | - | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha-Ramírez, L.M.; Hernández-Ochoa, B.; Gómez-Manzo, S.; Marcial-Quino, J.; Cárdenas-Rodríguez, N.; Centeno-Leija, S.; García-Garibay, M. Impact of Heat-Killed Lactobacillus casei Strain IMAU60214 on the Immune Function of Macrophages in Malnourished Children. Nutrients 2020, 12, 2303. https://doi.org/10.3390/nu12082303
Rocha-Ramírez LM, Hernández-Ochoa B, Gómez-Manzo S, Marcial-Quino J, Cárdenas-Rodríguez N, Centeno-Leija S, García-Garibay M. Impact of Heat-Killed Lactobacillus casei Strain IMAU60214 on the Immune Function of Macrophages in Malnourished Children. Nutrients. 2020; 12(8):2303. https://doi.org/10.3390/nu12082303
Chicago/Turabian StyleRocha-Ramírez, Luz María, Beatriz Hernández-Ochoa, Saúl Gómez-Manzo, Jaime Marcial-Quino, Noemí Cárdenas-Rodríguez, Sara Centeno-Leija, and Mariano García-Garibay. 2020. "Impact of Heat-Killed Lactobacillus casei Strain IMAU60214 on the Immune Function of Macrophages in Malnourished Children" Nutrients 12, no. 8: 2303. https://doi.org/10.3390/nu12082303
APA StyleRocha-Ramírez, L. M., Hernández-Ochoa, B., Gómez-Manzo, S., Marcial-Quino, J., Cárdenas-Rodríguez, N., Centeno-Leija, S., & García-Garibay, M. (2020). Impact of Heat-Killed Lactobacillus casei Strain IMAU60214 on the Immune Function of Macrophages in Malnourished Children. Nutrients, 12(8), 2303. https://doi.org/10.3390/nu12082303