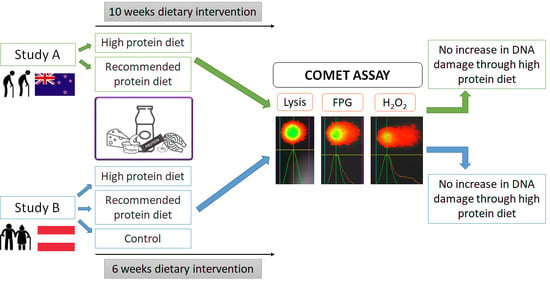
The Effect of Elevated Protein Intake on DNA Damage in Older People: Comparative Secondary Analysis of Two Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Austrian Study
2.1.1. Experimental Design Austrian Study
2.1.2. Participants Austrian Study
2.1.3. Nutritional Intervention Austrian Study
2.1.4. Dietary Intake Assessment Austrian Study
2.2. New Zealand Study
2.2.1. Experimental Design New Zealand Study
2.2.2. Participants New Zealand Study
2.2.3. Nutritional Intervention New Zealand Study
2.2.4. Dietary Intake Assessment New Zealand Study
2.3. Assessment of DNA Damage
2.4. Assessment of Oxidised and Reduced Glutathione
2.5. Anthropometry
2.6. Statistics
Statistical Analyses for Both Studies
3. Results
3.1. Baseline Characteristics and Nutrient Intake
3.1.1. Baseline Characteristics and Nutrient Intake Austrian Study
3.1.2. Baseline Characteristics and Nutrient Intake New Zealand Study
3.2. Macronutrient and Total Energy Intake
3.2.1. Macronutrient and Total Energy Intake Austrian Study
3.2.2. Macronutrient and Total Energy Intake New Zealand Study
3.3. Baseline Correlations between Markers of DNA Damage and Biochemical Parameter in the Austrian Study
3.4. Intervention Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Bernabei, R.; Onder, G.; Marzetti, E. Sarcopenia as the Biological Substrate of Physical Frailty. Clin. Geriatr. Med. 2015, 31, 367–374. [Google Scholar] [CrossRef]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef] [Green Version]
- Franzke, B.; Neubauer, O.; Cameron-Smith, D.; Wagner, K.H. Dietary Protein, Muscle and Physical Function in the Very Old. Nutrients 2018, 10, 935. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Biobaku, F.; Ghanim, H.; Batra, M.; Dandona, P. Macronutrient-Mediated Inflammation and Oxidative Stress: Relevance to Insulin Resistance, Obesity, and Atherogenesis. J. Clin. Endocrinol. Metab. 2019, 104, 6118–6128. [Google Scholar] [CrossRef]
- Grune, T. Oxidized protein aggregates: Formation and biological effects. Free Radic. Biol. Med. 2020, 150, 120–124. [Google Scholar] [CrossRef]
- Corbella, M.; Voityuk, A.A.; Curutchet, C. Single Amino Acid Mutation Controls Hole Transfer Dynamics in DNA-Methyltransferase HhaI Complexes. J. Phys. Chem. Lett. 2015, 6, 3749–3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hecke, T.; Vanden Bussche, J.; Vanhaecke, L.; Vossen, E.; Van Camp, J.; De Smet, S. Nitrite curing of chicken, pork, and beef inhibits oxidation but does not affect N-nitroso compound (NOC)-specific DNA adduct formation during in vitro digestion. J. Agric. Food Chem. 2014, 62, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Vossen, E.; Vanden Bussche, J.; Raes, K.; Vanhaecke, L.; De Smet, S. Fat content and nitrite-curing influence the formation of oxidation products and NOC-specific DNA adducts during in vitro digestion of meat. PLoS ONE 2014, 9, e101122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hecke, T.; Vossen, E.; Hemeryck, L.Y.; Vanden Bussche, J.; Vanhaecke, L.; De Smet, S. Increased oxidative and nitrosative reactions during digestion could contribute to the association between well-done red meat consumption and colorectal cancer. Food Chem. 2015, 187, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.; Ghanim, H.; Hamouda, W.; Aljada, A.; Garg, R.; Dandona, P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am. J. Clin. Nutr. 2002, 75, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Petzke, K.J.; Elsner, A.; Proll, J.; Thielecke, F.; Metges, C.C. Long-term high protein intake does not increase oxidative stress in rats. J. Nutr. 2000, 130, 2889–2896. [Google Scholar] [CrossRef]
- Pivovarova-Ramich, O.; Markova, M.; Weber, D.; Sucher, S.; Hornemann, S.; Rudovich, N.; Raila, J.; Sunaga-Franze, D.; Sauer, S.; Rohn, S.; et al. Effects of diets high in animal or plant protein on oxidative stress in individuals with type 2 diabetes: A randomized clinical trial. Redox Biol. 2020, 29, 101397. [Google Scholar] [CrossRef]
- Nabuco, H.C.G.; Tomeleri, C.M.; Fernandes, R.R.; Sugihara Junior, P.; Cavalcante, E.F.; Cunha, P.M.; Antunes, M.; Nunes, J.P.; Venturini, D.; Barbosa, D.S.; et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2019, 32, 88–95. [Google Scholar] [CrossRef]
- Shahar, S.; Kamaruddin, N.S.; Badrasawi, M.; Sakian, N.I.; Abd Manaf, Z.; Yassin, Z.; Joseph, L. Effectiveness of exercise and protein supplementation intervention on body composition, functional fitness, and oxidative stress among elderly Malays with sarcopenia. Clin. Interv. Aging 2013, 8, 1365–1375. [Google Scholar] [CrossRef] [Green Version]
- Franzke, B.; Halper, B.; Hofmann, M.; Oesen, S.; Jandrasits, W.; Baierl, A.; Tosevska, A.; Strasser, E.M.; Wessner, B.; Wagner, K.H.; et al. The impact of six months strength training, nutritional supplementation or cognitive training on DNA damage in institutionalised elderly. Mutagenesis 2015, 30, 147–153. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; 9789241208949; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Deutsche Gesellschaft für Ernährung (DGE) Österreichsiche Gesellschaft für Ernährung ÖGE), Schweizerische Gesellschaft für Ernährung (SGE). DACH Protein Referenzwerte. Available online: http://www.dge.de/wissenschaft/referenzwerte/protein/ (accessed on 20 July 2021).
- Huy, C. German-PAQ-50+—German-PAQ-50+ Fragebogen zur Erfassung der Körperlichen Aktivität. Z. Gerontol. Geriatr. 2011, 41, 209–216. [Google Scholar]
- Mitchell, C.J.; Milan, A.M.; Mitchell, S.M.; Zeng, N.; Ramzan, F.; Sharma, P.; Knowles, S.O.; Roy, N.C.; Sjödin, A.; Wagner, K.H.; et al. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: A 10-wk randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 1375–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillies, N.A.; Milan, A.M.; Chia, P.H.P.; Sharma, P.; Mitchell, S.M.; Zeng, N.; Ramzan, F.; D’Souza, R.F.; Mitchell, C.J.; Knowles, S.O.; et al. Responsiveness of one-carbon metabolites to a high-protein diet in older men: Results from a 10-wk randomized controlled trial. Nutrition 2021, 89, 111231. [Google Scholar] [CrossRef]
- Ministry of Health. Eating and Activity Guidelines for New Zealand Adults; Ministry of Health: Wellington, New Zealand, 2020.
- Grindel, A.; Guggenberger, B.; Eichberger, L.; Pöppelmeyer, C.; Gschaider, M.; Tosevska, A.; Mare, G.; Briskey, D.; Brath, H.; Wagner, K.H. Oxidative Stress, DNA Damage and DNA Repair in Female Patients with Diabetes Mellitus Type 2. PLoS ONE 2016, 11, e0162082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azqueta, A.; Shaposhnikov, S.; Collins, A.R. DNA oxidation: Investigating its key role in environmental mutagenesis with the comet assay. Mutat. Res. 2009, 674, 101–108. [Google Scholar] [CrossRef]
- Azqueta, A.; Gutzkow, K.B.; Priestley, C.C.; Meier, S.; Walker, J.S.; Brunborg, G.; Collins, A.R. A comparative performance test of standard, medium- and high-throughput comet assays. Toxicol. In Vitro 2013, 27, 768–773. [Google Scholar] [CrossRef]
- Al-Salmani, K.; Abbas, H.H.; Schulpen, S.; Karbaschi, M.; Abdalla, I.; Bowman, K.J.; So, K.K.; Evans, M.D.; Jones, G.D.; Godschalk, R.W.; et al. Simplified method for the collection, storage, and comet assay analysis of DNA damage in whole blood. Free Radic. Biol. Med. 2011, 51, 719–725. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Boon, A.C.; Hawkins, C.L.; Bisht, K.; Coombes, J.S.; Bakrania, B.; Wagner, K.H.; Bulmer, A.C. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic. Biol. Med. 2012, 52, 2120–2127. [Google Scholar] [CrossRef]
- Hofer, T.; Karlsson, H.L.; Möller, L. DNA oxidative damage and strand breaks in young healthy individuals: A gender difference and the role of life style factors. Free Radic. Res. 2006, 40, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Møller, P. Effect of age and sex on the level of DNA strand breaks and oxidatively damaged DNA in human blood cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2019, 838, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Bajpayee, M.; Dhawan, A.; Parmar, D.; Pandey, A.K.; Mathur, N.; Seth, P.K. Gender-related differences in basal DNA damage in lymphocytes of a healthy Indian population using the alkaline Comet assay. Mutat. Res. 2002, 520, 83–91. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef] [PubMed]
- Newson, L. Menopause and cardiovascular disease. Post Reprod. Health 2018, 24, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. Clinical effectiveness of protein and amino acid supplementation on building muscle mass in elderly people: A meta-analysis. PLoS ONE 2014, 9, e109141. [Google Scholar] [CrossRef] [Green Version]
- Geric, M.; Gajski, G.; Orešcanin, V.; Garaj-Vrhovac, V. Seasonal variations as predictive factors of the comet assay parameters: A retrospective study. Mutagenesis 2018, 33, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Moller, P.; Wallin, H.; Holst, E.; Knudsen, L.E. Sunlight-induced DNA damage in human mononuclear cells. FASEB J. 2002, 16, 45–53. [Google Scholar] [CrossRef]
- Weather in Januar 2017 in Auckland, New Zealand. Available online: https://www.timeanddate.com/weather/new-zealand/auckland/historic?month=1&year=2017 (accessed on 20 July 2021).
- Franzke, B.; Halper, B.; Hofmann, M.; Oesen, S.; Peherstorfer, H.; Krejci, K.; Koller, B.; Geider, K.; Baierl, A.; Tosevska, A.; et al. The influence of age and aerobic fitness on chromosomal damage in Austrian institutionalised elderly. Mutagenesis 2014, 29, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Milić, M.; Ceppi, M.; Bruzzone, M.; Azqueta, A.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.; Møller, P.; Teixeira, J.P.; et al. The hCOMET project: International database comparison of results with the comet assay in human biomonitoring. Baseline frequency of DNA damage and effect of main confounders. Mutat. Res. 2021, 787, 108371. [Google Scholar] [CrossRef]
| Total | CON | RP | HP | p-Value | |
|---|---|---|---|---|---|
| Sex [f/m], (% females)] | 73/63 (54.4%) | 24/23 (53.2%) | 21/20 (51.2%) | 28/20 (58.3%) | 0.724 |
| Age [years] | 72.9 ± 4.8 | 73.0 ± 4.9 | 72.4 ± 4.3 | 73.2 ± 5.2 | 0.732 |
| Body mass [kg] | 74.3 ± 13.6 | 74.3 ± 13.0 | 75.3 ± 15.3 | 73.4 ± 12.7 | 0.808 |
| Height [m] | 1.68 ± 0.1 | 1.69 ± 0.1 | 1.69 ± 0.1 | 1.67 ± 0.1 | 0.640 |
| BMI [kg/m2] | 26.2 ± 3.9 | 26.0 ± 4.0 | 26.3 ± 4.3 | 26.2 ± 3.6 | 0.938 |
| Energy intake [kcal/d] | 1845 ± 714 | 1838 ± 726 | 2000 ± 693 | 1719 ± 710 | 0.205 |
| Protein intake [g/kg BW/d] | 0.85 ± 0.43 | 0.83 ± 0.40 | 0.97 ± 0.55 | 0.78 ± 0.33 | 0.129 |
| Protein intake [g/d] | 62.3 ± 29.6 | 60.3 ± 27.8 | 71.6 ± 34.7 | 56.2 ± 24.8 | 0.052 |
| Fat intake [g/kg BW/d] | 1.07 ± 0.51 | 1.08 ± 0.56 | 1.12 ± 0.45 | 1.02 ± 0.53 | 0.681 |
| Fat intake [g/d] | 78.2 ± 39.1 | 78.2 ± 40.0 | 83.6 ± 37.5 | 73.8 ± 39.8 | 0.530 |
| Carbohydrate intake [g/kg BW/d] | 2.61 ± 1.24 | 2.68 ± 1.38 | 2.76 ± 1.09 | 2.43 ± 1.22 | 0.431 |
| Carbohydrate intake [g/d] | 188.5 ± 83.7 | 192.7 ± 89.0 | 204.9 ± 83.7 | 170.9 ± 76.9 | 0.171 |
| Lysis [%DNA in tail #] | 3.70 ± 1.48 | 3.66 ±1.65 | 3.67 ± 0.97 | 3.75 ± 1.68 | 0.953 |
| H2O2 [%DNA in tail #] | 10.67 ± 2.21 | 10.76± 2.28 | 10.03 ± 1.90 | 11.12 ± 2.30 | 0.067 |
| FPG [%DNA in tail #] | 7.40 ± 1.72 | 7.21 ± 1.70 | 7.34 ± 1.49 | 7.64 ± 1.93 | 0.483 |
| GSH [µmol/L] | 16.61 ± 3.68 | 15.94 ± 3.54 | 17.23 ±3.66 | 16.77 ± 3.81 | 0.255 |
| GSSG [µmol/L] | 8.40 ± 1.38 | 8.36 ± 1.47 | 8.34 ± 1.32 | 8.50 ± 1.37 | 0.830 |
| GSH:GSSG ratio [-] | 1.99 ± 0.35 | 1.92 ± 0.34 | 2.07 ± 0.31 | 1.99 ± 0.38 | 0.132 |
| Total-C [mmol/L] | 11.37 ± 2.30 | 11.03 ± 2.33 | 11.20 ± 1.96 | 11.85 ± 2.50 | 0.197 |
| LDL-C [mmol/L] | 6.52 ± 2.07 | 6.27 ± 2.01 | 6.38 ± 1.77 | 6.89 ± 2.33 | 0.308 |
| HDL-C [mmol/L] | 3.57 ± 0.92 | 3.43 ± 1.07 | 3.65 ± 0.84 | 3.63 ± 0.83 | 0.462 |
| TG [mmol/L] | 6.36 ± 2.75 | 6.49 ± 2.76 | 5.83 ± 2.52 | 6.68 ± 2.91 | 0.328 |
| TG-HDL-ratio | 2.00 ± 1.20 | 2.17 ± 1.29 | 1.78 ± 1.11 | 2.01 ± 1.17 | 0.336 |
| CRP [mg/L] | 2.18 ± 2.40 | 1.55 ± 1.57 * | 3.05 ± 3.09 * | 2.08 ± 2.24 | 0.013 |
| Total | RDA | 2RDA | p-Value | |
|---|---|---|---|---|
| Age [years] | 74.2 ± 3.6 | 74.7 ± 3.9 | 73.7 ± 3.3 | 0.342 |
| Body mass [kg] | 84.2 ± 15.6 | 85.3 ± 20.1 | 82.5 ± 7.9 | 0.320 |
| Height [m] | 1.72 ± 0.07 | 1.73 ± 0.08 | 1.72 ± 0.06 | 0.874 |
| BMI [kg/m2] | 28.2 ± 4.2 | 28.4 ± 5.1 | 28.2 ± 3.3 | 0.268 |
| Energy intake [kcal/d] | 2316 ± 523 | 2488 ± 460 | 2132 ± 539 | 0.068 |
| Protein intake [g/kg BW/d] | 1.23 ± 0.28 | 1.26 ± 0.27 | 1.20 ± 0.29 | 0.616 |
| Protein intake [g/d] | 102.1 ± 23.9 | 107.3 ± 26.4 | 96.6 ± 20.4 | 0.234 |
| Fat intake [g/kg BW/d] | 1.09 ± 0.34 | 1.17 ± 0.31 | 1.01 ± 0.37 | 0.218 |
| Fat intake [g/d] | 89.9 ± 25.8 | 99.1 ± 26.0 | 80.0 ± 22.3 | 0.043 |
| Carbohydrate intake [g/kg BW/d] | 3.00 ± 1.15 | 3.10 ± 0.92 | 2.89 ± 1.38 | 0.637 |
| Carbohydrate intake [g/d] | 243.3 ± 76.7 | 260.0 ± 62.0 | 225.4 ± 88.6 | 0.238 |
| Lysis [%DNA in tail #] | 4.72 ± 3.21 | 4.37 ± 2.91 | 5.10 ± 3.58 | 0.547 |
| H2O2 [%DNA in tail #] | 4.91 ± 2.39 | 4.73 ± 2.76 | 5.11 ± 2.00 | 0.677 |
| FPG [%DNA in tail #] | 3.37 ± 1.95 | 7.20 ± 1.72 | 3.27 ± 2.31 | 0.600 |
| GSH [µmol/L] | 17.46 ± 3.63 | 18.13 ± 3.91 | 16.73 ± 3.30 | 0.308 |
| GSSG [µmol/L] | 6.51 ± 1.91 | 6.54 ± 2.30 | 6.49 ± 1.48 | 0.948 |
| GSH:GSSG ratio | 2.96 ± 1.30 | 3.11 ± 11.37 | 2.80 ± 1.23 | 0.528 |
| Total-C [mmol/L] | 4.64 ± 0.88 | 4.77 ± 1.04 | 4.52 ± 0.71 | 0.445 |
| LDL-C [mmol/L] | 2.95 ± 0.91 | 2.81 ± 0.76 | 3.09 ± 1.06 | 0.428 |
| HDL-C [mmol/L] | 1.34 ± 0.40 | 1.34 ±0.44 | 1.35 ± 0.37 | 0.941 |
| TG [mmol/L] | 1.13 ± 0.52 | 1.17 ± 0.39 | 1.08 ± 0.64 | 0.654 |
| TG-HDL_ratio | 0.97 ± 0.70 | 1.00 ± 0.51 | 0.94 ± 0.88 | 0.816 |
| CRP [mg/L] | 1.98 ± 2.49 | 1.63 ± 2.09 | 2.18 ± 2.92 | 0.564 |
| Parameter | Group | Pre (t1) | Post (t2) | Time p-Value | Group p-Value | Time × Group p-Value |
|---|---|---|---|---|---|---|
| Protein [g/kg BW/d] | CON | 0.83 ± 0.40 | 0.91 ± 0.36 | <0.001 | <0.001 | <0.001 |
| RP | 0.89 ± 0.28 | 1.08 ± 0.32 ** | ||||
| HP | 0.79 ± 0.31 | 1.54 ± 0.35 *** | ||||
| Carbohydrates [g/kg BW/d] | CON | 2.68 ± 1.38 | 2.70 ± 1.10 | 0.566 | 0.891 | 0.408 |
| RP | 2.72 ± 1.11 | 2.83 ± 0.95 | ||||
| HP | 2.46 ± 1.21 | 2.48 ± 0.88 | ||||
| Fat [g/kg BW/d] | CON | 1.08 ± 0.56 | 1.18 ± 0.44 | 0.100 | 0.699 | 0.876 |
| RP | 1.08 ± 0.39 | 1.12 ± 0.36 | ||||
| HP | 1.03 ± 0.52 | 1.09 ± 0.38 | ||||
| Energy intake [kcal/d] | CON | 1838 ± 726 | 1961 ± 578 | 0.001 | 0.475 | 0.332 |
| RP | 1981 ± 690 | 2118 ± 630 | ||||
| HP | 1730 ± 700 | 1989 ± 525 ** |
| Parameter | Group | Pre (t1) | Post (t2) | Time p-Value | Group p-Value | Time × Group p-Value |
|---|---|---|---|---|---|---|
| Protein [g/kg BW/d] | RDA | 1.26 ± 0.27 | 0.89 ± 0.09 *** | 0.164 | <0.001 | <0.001 |
| 2RDA | 1.20 ± 0.29 | 1.74 ± 0.22 *** | ||||
| Carbohydrates [g/kg BW/d] | RDA | 3.10 ± 0.92 | 4.18 ± 0.83 ** | <0.001 | 0.794 | 0.397 |
| 2RDA | 2.89 ± 1.38 | 4.21 ± 0.89 *** | ||||
| Fat [g/kg BW/d] | RDA | 1.17 ± 0.31 | 1.11 ± 0.23 | 0.706 | 0.359 | 0.164 |
| 2RDA | 1.01 ± 0.37 | 1.12 ± 0.18 | ||||
| Energy intake [kcal/d] | RDA | 2488 ± 460 | 2683 ± 516 | <0.001 | 0.423 | 0.009 |
| 2RDA | 2132 ± 539 | 2823 ± 153 *** |
| Biomarker | Lysis [%DNA in Tail] | H2O2 [%DNA in Tail] | FPG [%DNA in Tail] |
|---|---|---|---|
| Total-C [mg/dL] | 0.472 ** | 0.256 ** | 0.394 ** |
| HDL-C [mg/dL] | −0.243 ** | −0.169 | −0.027 |
| LDL-C [mg/dL] | 0.462 ** | 0.265 ** | 0.377 ** |
| TG [mg/dL] | 0.615 ** | 0.365 ** | 0.281 ** |
| Total-C/HDL-C | 0.600 ** | 0.364 ** | 0.296 ** |
| Parameter | Group | Mean ± Stdv | Time | Group | Time × Group | Time Points Differences | |
|---|---|---|---|---|---|---|---|
| Baseline | 6 Weeks | p-Value | p-Value | p-Value | Δ (Post–Pre) | ||
| Lysis [%DNA in tail] | CON | 3.74 ± 1.73 | 3.28 ± 1.19 *** | 0.002 | 0.446 | 0.263 | −0.45 ± 1.24 |
| RP | 3.64 ± 0.99 | 3.54 ± 1.32 | −0.10 ± 1.51 | ||||
| HP | 3.55 ± 1.17 | 3.03 ± 0.84 *** | −0.53 ± 0.82 | ||||
| H2O2 [%DNA in tail] | CON | 10.92 ± 2.35 | 9.69 ± 2.06 *** | <0.001 | 0.363 | 0.156 | −1.23 ± 1.83 |
| RP | 10.00 ± 1.89 | 9.43 ± 1.92 | −0.58 ± 1.96 | ||||
| HP | 10.73 ± 2.02 | 9.40 ± 1.94 *** | −1.33 ± 1.74 | ||||
| FPG [%DNA in tail] | CON | 7.23 ± 1.79 | 6.33 ± 1.51 *** | <0.001 | 0.698 | 0.148 | −1.15 ± 1.61 |
| RP | 7.32 ± 1.49 | 6.78 ± 1.91 ** | −0.54 ± 1.96 | ||||
| HP | 7.45 ± 1.93 | 6.18 ± 1.64 *** | −1.54 ± 1.67 | ||||
| GSH [µmol/L] | CON | 15.98 ± 3.71 | 15.58 ± 2.69 | 0.002 | 0.373 | 0.417 | −0.40 ± 2.91 |
| RP | 17.34 ± 3.69 | 16.10 ± 2.91 * | −1.24 ± 2.86 | ||||
| HP | 16.74 ± 3.93 | 15.96 ± 2.35 * | −0.77 ± 2.54 | ||||
| GSSG [µmol/L] | CON | 8.23 ± 1.47 | 8.17 ± 1.46 | 0.172 | 0.831 | 0.764 | −0.06 ± 1.61 |
| RP | 8.40 ± 1.35 | 8.21 ± 1.49 | −0.20 ± 1.29 | ||||
| HP | 8.51 ± 1.45 | 8.22 ± 1.09 | −0.29 ± 1.37 | ||||
| GSH:GSSG ratio | CON | 1.95 ± 0.34 | 1.95 ± 0.37 | 0.302 | 0.440 | 0.727 | −0.001 ± 0.43 |
| RP | 2.07 ± 0.31 | 2.00 ± 0.39 | −0.07 ± 0.33 | ||||
| HP | 1.99 ± 0.39 | 1.96 ± 0.28 | −0.03 ± 0.28 | ||||
| CRP [mg/L] | CON | 1.48 ± 1.47 | 2.62 ± 6.79 | 0.207 | 0.459 | 0.385 | 1.15 ± 6.71 |
| RP | 3.10 ± 3.21 | 2.76 ± 2.67 | −0.34 ± 3.30 | ||||
| HP | 2.05 ± 2.31 | 3.03 ± 5.09 | 0.99 ± 4.64 | ||||
| Parameter | Group | Mean ± Stdv | Time | Group | Time × Group | Time Points Differences | |
|---|---|---|---|---|---|---|---|
| Baseline | 10 Weeks | p-Value | p-Value | p-Value | Δ (post–pre) | ||
| Lysis [%DNA in tail] | RDA | 5.10 ± 3.58 | 4.62 ± 3.16 | 0.72 | 0.729 | 0.348 | 0.22 ± 1.68 |
| 2RDA | 4.37 ± 2.91 | 4.58 ± 2.94 | −0.48 ± 2.22 | ||||
| H2O2 [%DNA in tail] | RDA | 5.11± 2.00 | 4.90 ± 2.71 | 0.608 | 0.643 | 0.999 | −0.21 ± 2.06 |
| 2RDA | 4.73 ± 2.76 | 4.52 ± 2.14 | −0.21 ± 2.26 | ||||
| FPG [%DNA in tail] | RDA | 3.27 ± 2.31 | 3.36 ± 1.81 | 0.869 | 0.614 | 0.994 | 0.09 ± 2.68 |
| 2RDA | 3.46 ± 1.64 | 3.54 ± 2.40 | 0.09 ± 3.10 | ||||
| GSH [µmol/L] | RDA | 16.73 ± 3.30 | 16.37 ± 1.26 | 0.059 | 0.475 | 0.189 | −1.24 ± 2.86 |
| 2RDA | 18.13 ± 3.91 | 16.24 ± 1.87 | −0.72 ± 2.54 | ||||
| GSSG [µmol/L] | RDA | 6.49 ± 1.48 | 6.87 ± 1.72 | 0.657 | 0.796 | 0.518 | −0.07 ± 2.02 |
| 2RDA | 6.57 ± 2.30 | 6.47 ± 2.43 | 0.38 ± 1.61 | ||||
| GSH:GSSG ratio | RDA | 2.80 ± 1.23 | 2.66 ± 1.34 | 0.146 | 0.806 | 0.545 | −0.32 ± 1.05 |
| 2RDA | 3.11 ± 1.38 | 2.78 ± 0.87 | −0.14 ± 0.50 | ||||
| CRP [mg/L] | RDA | 2.18± 2.92 | 2.87 ± 3.34 | 0.71 | 0.281 | 0.28 | −0.34 ± 2.63 |
| 2RDA | 1.74 ± 2.23 | 1.39 ± 1.08 | 0.70 ± 2.27 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Draxler, A.; Franzke, B.; Cortolezis, J.T.; Gillies, N.A.; Unterberger, S.; Aschauer, R.; Zöhrer, P.A.; Bragagna, L.; Kodnar, J.; Strasser, E.-M.; et al. The Effect of Elevated Protein Intake on DNA Damage in Older People: Comparative Secondary Analysis of Two Randomized Controlled Trials. Nutrients 2021, 13, 3479. https://doi.org/10.3390/nu13103479
Draxler A, Franzke B, Cortolezis JT, Gillies NA, Unterberger S, Aschauer R, Zöhrer PA, Bragagna L, Kodnar J, Strasser E-M, et al. The Effect of Elevated Protein Intake on DNA Damage in Older People: Comparative Secondary Analysis of Two Randomized Controlled Trials. Nutrients. 2021; 13(10):3479. https://doi.org/10.3390/nu13103479
Chicago/Turabian StyleDraxler, Agnes, Bernhard Franzke, Johannes T. Cortolezis, Nicola A. Gillies, Sandra Unterberger, Rudolf Aschauer, Patrick A. Zöhrer, Laura Bragagna, Julia Kodnar, Eva-Maria Strasser, and et al. 2021. "The Effect of Elevated Protein Intake on DNA Damage in Older People: Comparative Secondary Analysis of Two Randomized Controlled Trials" Nutrients 13, no. 10: 3479. https://doi.org/10.3390/nu13103479
APA StyleDraxler, A., Franzke, B., Cortolezis, J. T., Gillies, N. A., Unterberger, S., Aschauer, R., Zöhrer, P. A., Bragagna, L., Kodnar, J., Strasser, E. -M., Neubauer, O., Sharma, P., Mitchell, S. M., Zeng, N., Ramzan, F., D’Souza, R. F., Knowles, S. O., Roy, N. C., Sjödin, A. M., ... Wagner, K. -H. (2021). The Effect of Elevated Protein Intake on DNA Damage in Older People: Comparative Secondary Analysis of Two Randomized Controlled Trials. Nutrients, 13(10), 3479. https://doi.org/10.3390/nu13103479