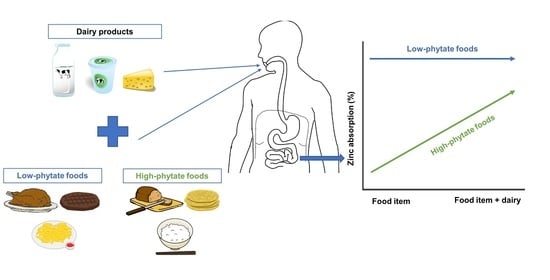
Influence of Dairy Products on Bioavailability of Zinc from Other Food Products: A Review of Complementarity at a Meal Level
Abstract
:1. Introduction
2. Zinc in Human Nutrition
3. Zinc Absorption
4. Zinc Absorption from Dairy Products
5. Zinc Absorption from Meals Containing Dairy Products
6. Factors Affecting Zinc Absorption from Meals Containing Dairy Products
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant-And animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R.; Baum, J.I.; Starck, C.; Moughan, P.J. Factors contributing to the selection of dietary protein food sources. Clin. Nutr. 2018, 37, 130–138. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Rutherfurd, S.M.; Kim, I.Y.; Moughan, P.J. Protein quality as determined by the Digestible Indispensable Amino Acid Score: Evaluation of factors underlying the calculation. Nutr. Rev. 2016, 74, 584–599. [Google Scholar] [CrossRef]
- Reynaud, Y.; Buffière, C.; Cohade, B.; Vauris, M.; Liebermann, K.; Hafnaoui, N.; Lopez, M.; Souchon, I.; Dupont, D.; Rémond, D. True ileal amino acid digestibility and digestible indispensable amino acid scores (DIAASs) of plant-based protein foods. Food Chem. 2021, 338, 128020. [Google Scholar] [CrossRef]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, N.S.; Bailey, H.M.; Guardiola, L.V.; Stein, H.H. Values for Digestible Indispensable Amino Acid Score (DIAAS) Determined in Pigs Are Greater for Milk Than for Breakfast Cereals, but DIAAS Values for Individual Ingredients Are Additive in Combined Meals. J. Nutr. 2021, 151, 540–547. [Google Scholar] [CrossRef]
- Kashyap, S.; Shivakumar, N.; Varkey, A.; Preston, T.; Devi, S.; Kurpad, A.V. Co-ingestion of black tea reduces the indispensable amino acid digestibility of hens’ egg in Indian adults. J. Nutr. 2019, 149, 1363–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The role of dietary phenolic compounds in protein digestion and processing technologies to improve their antinutritive properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef]
- Gibson, R.S.; Raboy, V.; King, J.C. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr. Rev. 2018, 76, 793–804. [Google Scholar] [CrossRef]
- Castro-Alba, V.; Lazarte, C.E.; Bergenståhl, B.; Granfeldt, Y. Phytate, iron, zinc, and calcium content of common Bolivian foods and their estimated mineral bioavailability. Food Sci. Nutr. 2019, 7, 2854–2865. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.; Fransen, H.; Jenab, M.; Boutron-Ruault, M.; Tumino, R.; Agnoli, C.; Ericson, U.; Johansson, I.; Ferrari, P.; Engeset, D. Variation in intakes of calcium, phosphorus, magnesium, iron and potassium in 10 countries in the European Prospective Investigation into Cancer and Nutrition study. Eur. J. Clin. Nutr. 2009, 63, S101–S121. [Google Scholar] [CrossRef] [Green Version]
- Guéguen, L.; Pointillart, A. The bioavailability of dietary calcium. J. Am. Coll. Nutr. 2000, 19, 119S–136S. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. A guide to human zinc absorption: General overview and recent advances of in vitro intestinal models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mawson, C.A.; Fischer, M.I. The occurrence of zinc in the human prostate gland. Can. J. Appl. Sci. 1952, 30, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Driessen, C.; Hirv, K.; Kirchner, H.; Rink, L. Zinc regulates cytokine induction by superantigens and lipopolysaccharide. Immunology 1995, 84, 272. [Google Scholar] [PubMed]
- Hess, S.Y.; Peerson, J.M.; King, J.C.; Brown, K.H. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr. Bull. 2007, 28, S403–S429. [Google Scholar] [CrossRef]
- Wessells, K.R.; Jorgensen, J.M.; Hess, S.Y.; Woodhouse, L.R.; Peerson, J.M.; Brown, K.H. Plasma zinc concentration responds rapidly to the initiation and discontinuation of short-term zinc supplementation in healthy men. J. Nutr. 2010, 140, 2128–2133. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Smoak, B.L.; Patterson, K.Y.; LeMay, L.G.; Veillon, C.; Deuster, P. Biochemical indices of selected trace minerals in men: Effect of stress. Am. J. Clin. Nutr. 1991, 53, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Scott, B.J.; Bradwell, A.R. Identification of the serum binding proteins for iron, zinc, cadmium, nickel, and calcium. Clin. Chem. 1983, 29, 629–633. [Google Scholar] [CrossRef]
- Prasad, A.S.; Halsted, J.A.; Nadimi, M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am. J. Med. 1961, 31, 532–546. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef]
- Sandstead, H.H. Zinc nutrition from discovery to global health impact. Adv. Nutr. 2012, 3, 718–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, C. Zinc: Physiology, deficiency, and parenteral nutrition. Nutr. Clin. Prac. 2015, 30, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Nour, Z.G.; Lothar, R. Zinc in infection and inflammation. Nutrients 2017, 9, 624. [Google Scholar]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Dietary Reference Values for Nutrients: Summary Report; EFSA Supporting Publications: Parma, Italy, 2017. [Google Scholar]
- Gibson, R.S. A historical review of progress in the assessment of dietary zinc intake as an indicator of population zinc status. Adv. Nutr. 2012, 3, 772–782. [Google Scholar] [CrossRef] [Green Version]
- Roohani, N.; Hurrell, R.; Wegmueller, R.; Schulin, R. Zinc and phytic acid in major foods consumed by a rural and a suburban population in central Iran. J. Food Comp. Anal. 2012, 28, 8–15. [Google Scholar] [CrossRef]
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc absorption in human small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 1989, 256, G87–G91. [Google Scholar] [CrossRef]
- Sandström, B. Dose dependence of zinc and manganese absorption in man. Proc. Nutr. Soc. 1992, 51, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-zinc review. J. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef] [Green Version]
- Steinhardt, H.J.; Adibi, S.A. Interaction between transport of zinc and other solutes in human intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 1984, 247, G176–G182. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144. [Google Scholar]
- McLoughlin, I.; Hodge, J. Zinc in depressive disorder. Acta Psychiatr. Scand. 1990, 82, 451–453. [Google Scholar] [CrossRef]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [CrossRef] [Green Version]
- Cousins, R.J.; Liuzzi, J.P.; Lichten, L.A. Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 2006, 281, 24085–24089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousins, R.J. Gastrointestinal factors influencing zinc absorption and homeostasis. Int. J. Vitam. Nutr. Res. 2010, 80, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeejeebhoy, K. Zinc: An essential trace element for parenteral nutrition. Gastroenterology 2009, 137, S7–S12. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Turnlund, J.; King, J.; Keyes, W.; Gong, B.; Michel, M. A stable isotope study of zinc absorption in young men: Effects of phytate and a-cellulose. Am. J. Clin. Nutr. 1984, 40, 1071–1077. [Google Scholar] [CrossRef]
- Cousins, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Sandström, B.; Cederblad, A. Zinc absorption from composite meals II. Influence of the main protein source. Am. J. Clin. Nutr. 1980, 33, 1778–1783. [Google Scholar] [CrossRef]
- Sauer, A.K.; Pfaender, S.; Hagmeyer, S.; Tarana, L.; Mattes, A.-K.; Briel, F.; Küry, S.; Boeckers, T.M.; Grabrucker, A.M. Characterization of zinc amino acid complexes for zinc delivery in vitro using Caco-2 cells and enterocytes from hiPSC. Biometals 2017, 30, 643–661. [Google Scholar] [CrossRef] [Green Version]
- Van Rossum, C.; Buurma-Rethans, E.; Dinnissen, C.; Beukers, M.; Brants, H.; Dekkers, A.; Ocké, M. The Diet of the Dutch: Results of the Dutch National Food Consumption Survey 2012–2016; RIVM Report 2020-0083; RIVM: Bilthoven, The Netherlands, 2020. [Google Scholar]
- Gaucheron, F. Milk minerals, trace elements, and macroelements. Milk Dairy Prod. Hum. Nutr. Prod. Compos. Health 2013, 172–199. [Google Scholar] [CrossRef]
- Sandström, B.; Cederblad, Å.; Lönnerdal, B. Zinc absorption from human milk, cow’s milk, and infant formulas. Am. J. Dis. Child. 1983, 137, 726–729. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Cederblad, Å.; Davidsson, L.; Sandström, B. The effect of individual components of soy formula and cows’ milk formula on zinc bioavailability. Am. J. Clin. Nutr. 1984, 40, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Talsma, E.F.; Moretti, D.; Ly, S.C.; Dekkers, R.; van den Heuvel, E.G.; Fitri, A.; Boelsma, E.; Stomph, T.J.; Zeder, C.; Melse-Boonstra, A. Zinc absorption from milk is affected by dilution but not by thermal processing, and milk enhances absorption of zinc from high-phytate rice in young Dutch women. J. Nutr. 2017, 147, 1086–1093. [Google Scholar] [CrossRef] [Green Version]
- Galetti, V.; Kujinga, P.; Mitchikpe, C.E.S.; Zeder, C.; Tay, F.; Tossou, F.; Hounhouigan, J.D.; Zimmermann, M.B.; Moretti, D. Efficacy of highly bioavailable zinc from fortified water: A randomized controlled trial in rural Beninese children. Am. J. Clin. Nutr. 2015, 102, 1238–1248. [Google Scholar] [CrossRef] [Green Version]
- Hotz, C.; Brown, K.H. Assessment of the risk of zinc deficiency in populations and options for its control. Food. Nutr. Bull. 2004, 25, 94–204. [Google Scholar]
- Rosado, J.L.; Díaz, M.; Gonzalez, K.; Griffin, I.; Abrams, S.A.; Preciado, R. The addition of milk or yogurt to a plant-based diet increases zinc bioavailability but does not affect iron bioavailability in women. J. Nutr. 2005, 135, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B.; Arvidsson, B.; Cederblad, A.; Björn-Rasmussen, E. Zinc absorption from composite meals I. The significance of wheat extraction rate, zinc, calcium, and protein content in meals based on bread. Am. J. Clin. Nutr. 1980, 33, 739–745. [Google Scholar] [CrossRef]
- Hunt, J.R.; Lykken, G.I.; Mullen, L.K. Moderate and high amounts of protein from casein enhance human absorption of zinc from whole-wheat or white rolls. Nutr. Res. 1991, 11, 413–418. [Google Scholar] [CrossRef]
- Pecoud, A.; Donzel, P.; Schelling, J. Effect of foodstuffs on the absorption of zinc sulfate. Clin. Pharmacol. Ther. 1975, 17, 469–474. [Google Scholar] [CrossRef]
- Flanagan, P.R.; Cluett, J.; Chamberlain, M.J.; Valberg, L.S. Dual-isotope method for determination of human zinc absorption: The use of a test meal of turkey meat. J. Nutr. 1985, 115, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Zheng, J.J. Milk consumption and zinc retention in postmenopausal women. J. Nutr. 1990, 120, 398–403. [Google Scholar] [CrossRef]
- Solomons, N.W. Dietary sources of zinc and factors affecting its bioavailability. Food Nutr. Bull. 2001, 22, 138–154. [Google Scholar] [CrossRef]
- Della Lucia, C.M.; Santos, L.L.M.; da Cruz Rodrigues, K.C.; da Cruz Rodrigues, V.C.; Martino, H.S.D.; Sant’Ana, H.M.P. Bioavailability of zinc in Wistar rats fed with rice fortified with zinc oxide. Nutrients 2014, 6, 2279–2289. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Zinc bioavailability from phytate-rich foods and zinc supplements. Modeling the effects of food components with oxygen, nitrogen, and sulfur donor ligands. J. Agric. Food Chem. 2017, 65, 8727–8743. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar]
- Allen, L.; De Benoist, B.; Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; WHO/FAO: Geneva, Switzerland, 2006. [Google Scholar]
- Oberleas, D. The role of phytate in zinc bioavailability and homeostasis. In Nutritional Bioavailability of Zinc; Inglett, G.E., Ed.; American Chemical Society: Washington, DC, USA, 1983; pp. 145–158. [Google Scholar]
- Linzell, J.; Mepham, T.; Peaker, M. The secretion of citrate into milk. J. Physiol. 1976, 260, 739–750. [Google Scholar] [CrossRef]
- Pabón, M.L.; Lönnerdal, B. Effect of citrate on zinc bioavailability from milk, milk fractions and infant formulas. Nutr. Res. 1993, 13, 103–111. [Google Scholar] [CrossRef]
- Hansen, M.; Sandström, B.; Jensen, M.; Sørensen, S. Effect of casein phosphopeptides on zinc and calcium absorption from bread meals. J. Trace Elem. Med. Biol. 1997, 11, 143–149. [Google Scholar] [CrossRef]
- Forbes, R.M.; Erdman, J.W., Jr.; Parker, H.M.; Kondo, H.; Ketelsen, S.M. Bioavailability of zinc in coagulated soy protein (tofu) to rats and effect of dietary calcium at a constant phytate: Zinc ratio. J. Nutr. 1983, 113, 205–210. [Google Scholar] [CrossRef]
- Bosscher, D.; Lu, Z.; Janssens, G.; Van Caillie-Bertrand, M.; Robberecht, H.; De Rycke, H.; De Wilde, R.; Deelstra, H. In vitro availability of zinc from infant foods with increasing phytic acid contents. Br. J. Nutr. 2001, 86, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wapnir, R.A.; Khani, D.E.; Bayne, M.A.; Lifshitz, F. Absorption of zinc by the rat ileum: Effects of histidine and other low-molecular-weight ligands. J. Nutr. 1983, 113, 1346–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miquel, E.; Farré, R. Effects and future trends of casein phosphopeptides on zinc bioavailability. Trends Food Sci. Technol. 2007, 18, 139–143. [Google Scholar] [CrossRef]
- Reynolds, E.C. Phosphopeptides for the Treatment of Dental Calculus. World Patent WO 93/03707, 4 March 1993. [Google Scholar]
- Hansen, M.; Sandström, B.; Jensen, M.; Sørensen, S.S. Casein phosphopeptides improve zinc and calcium absorption from rice-based but not from whole-grain infant cereal. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Sandström, B.; Lönnerdal, B. The effect of casein phosphopetides on zinc and calcium absorption from high phytate infant diets assessed in rat pups and Caco-2 cells. Pediatr. Res. 1996, 40, 547–552. [Google Scholar] [CrossRef] [Green Version]
| Meals | Zinc Intake (mg) | Zinc Absorption (%) 1 | Significance | Methods | References |
|---|---|---|---|---|---|
| 450 mL Human Milk (Casein:Whey Ratio, 40:60) (Control) | 1.3 | 41.0 ± 2.3 | |||
| 450 mL Bovine Milk, 3% Fat (Casein:Whey Ratio, 80:20) | 1.6 | 28.0 ± 6.1 | S | Radioisotope | [50] |
| 450 mL Humanized Bovine Milk Formula (Casein:Whey Ratio, 40:60) | 1.2 | 31.0 ± 1.8 | S | ||
| 450 mL soy Protein-Isolate Formula | 1.7 | 14.0 ± 1.4 | S | ||
| 450 mL Bovine Milk Formula (Whey:Casein 60:40) (Control) | 1.2 | 32.2 ± 1.4 | |||
| 450 mL Bovine Milk Formula (Whey:Casein 20:80) | 3.2 | 21.3 ± 2.9 | S | Radioisotope | [51] |
| Water (Control) | 4.22 | 72.3 ± 1.7 | Dual stable isotopes | [52] | |
| Bovine Milk | 4.04 | 25.5 ± 1.8 | S |
| Meals | Zinc Intake (mg) | Zinc Absorption (%) 1 | Significance | Methods | References |
|---|---|---|---|---|---|
| 90 g Cooked Rice + 600 mL Water (Control) | 3.8 | 12.8 ± 0.9 | Dual stable isotopes | [52] | |
| 90 g Cooked Rice + 600 mL Milk full Fat UHT | 3.6 | 20.8 ± 0.9 | S | ||
| Plant-Based Test Meal (Control) | 4.8 | 7.1 ± 1.2 | |||
| Plant-Based Test Meal + 250 mL of Milk | 5.8 | 10.6 ± 1.2 | S | Dual stable isotopes | [55] |
| Plant-Based Test Meal + 150 g of Yogurt | 5.7 | 11.9 ± 1.2 | S | ||
| 60 g Wholemeal Bread (Control) | 3.5 | 8.2 (5.7–11.3) | |||
| 60 g Wholemeal Bread + 200 g Milk | 3.1 | 9.9 (5.6–14.4) | Radioisotope | [56] | |
| 60 g Wholemeal Bread + 200 g Milk + 42 g Cheese | 3.2 | 14 (8.7–21.8) | S | ||
| Rolls with White Flour + 8.9 g Casein (Control) | 4.1 | 13.0 ± 0.6 | |||
| Rolls with White Flour + 50.5 g Casein | 3.9 | 26.0 ± 2.2 | S | Radioisotope | [57] |
| Rolls with Whole Wheat Flour + 10.6 g Casein (Control) | 3.9 | 8.0 ± 1.3 | |||
| Rolls with Whole-Wheat Flour + 51.7 g Casein | 4.0 | 25.0 ± 2.2 | S | ||
| Soybean Meals (Control) | 2.5 | 19.6 ± 2.0 | Radioisotope | [46] | |
| Soybean Meals + 125 mL Milk | 2.7 | 14.1 ± 0.5 | S | ||
| Turkey Meal + 250 mL Deionized Water (Control) | 4.0 | 29.0 ± 2.2 | Dual isotopes | [58] | |
| Turkey Meal + 210 mL Milk | 4.0 | 22.0 ± 2.5 | NS | ||
| Basal Diets (Control) | 14.5 | 22.0 ± 6 | Dual isotopes | [59] | |
| Basal Diets + 400 mL Milk Per Day | 16.0 | 23.0 ± 6 | NS |
| Meals | Zinc Intake (mg) | Zinc Absorption (%) 1 | Significance | Methods | References |
|---|---|---|---|---|---|
| Rice-Based Cereal + 0 g CPP | 1.29 | 19.4 ± 2.7 | |||
| Rice-Based Cereal + 1 g CPP | 1.29 | 25.2 ± 2.3 | S | ||
| Rice-Based Cereal + 2 g CPP | 1.29 | 23.9 ± 1.6 | S | Radioisotope | [75] |
| Whole Grain Cereal + 0 g CPP | 1.77 | 16.0 ± 1.5 | |||
| Whole Grain Cereal + 1 g CPP | 1.77 | 15.3 ± 0.9 | NS | Radioisotope | [75] |
| Whole Grain Cereal + 2 g CPP | 1.77 | 18.1 ± 1.3 | NS | ||
| Bread Meal, High-Phytate/High Calcium + 0 mg CPP | 1.4 | 7.0 ± 0.5 | |||
| Bread Meal, High-Phytate/High Calcium + 250 mg CPP | 1.4 | 7.7 ± 0.9 | NS | Radioisotope | [75] |
| Bread Meal, High-Phytate/High Calcium + 1000 mg CPP | 1.4 | 8.0 ± 0.8 | NS | ||
| Bread Meal, High-Phytate/Low Calcium + 0 mg CPP | 1.3 | 7.7 ± 0.8 | |||
| Bread Meal, High-Phytate/Low Calcium + 250 mg CPP | 1.3 | 7.0 ± 0.7 | NS | Radioisotope | [69] |
| Bread Meal, High-Phytate/Low Calcium + 1000 mg CPP | 1.3 | 6.5 ± 0.5 | NS | ||
| Bread Meal, Low-Phytate/High Calcium + 0 mg CPP | 1.5 | 14.3 ± 1.4 | |||
| Bread Meal, Low-Phytate/High Calcium + 250 mg CPP | 1.5 | 16.7 ± 2.1 | NS | Radioisotope | [69] |
| Bread Meal, Low-Phytate/High Calcium + 1000 mg CPP | 1.5 | 16.0 ± 2.8 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkembi, B.; Huppertz, T. Influence of Dairy Products on Bioavailability of Zinc from Other Food Products: A Review of Complementarity at a Meal Level. Nutrients 2021, 13, 4253. https://doi.org/10.3390/nu13124253
Shkembi B, Huppertz T. Influence of Dairy Products on Bioavailability of Zinc from Other Food Products: A Review of Complementarity at a Meal Level. Nutrients. 2021; 13(12):4253. https://doi.org/10.3390/nu13124253
Chicago/Turabian StyleShkembi, Blerina, and Thom Huppertz. 2021. "Influence of Dairy Products on Bioavailability of Zinc from Other Food Products: A Review of Complementarity at a Meal Level" Nutrients 13, no. 12: 4253. https://doi.org/10.3390/nu13124253
APA StyleShkembi, B., & Huppertz, T. (2021). Influence of Dairy Products on Bioavailability of Zinc from Other Food Products: A Review of Complementarity at a Meal Level. Nutrients, 13(12), 4253. https://doi.org/10.3390/nu13124253