Unhealthy Lifestyle, Genetics and Risk of Cardiovascular Disease and Mortality in 76,958 Individuals from the UK Biobank Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Lifestyle Behaviours
2.3. Diet Quality
2.4. Other Lifestyle Behaviours
2.5. Polygenic Risk Score
2.6. Cardiovascular Events and Mortality
2.7. Demographic and Health Information
2.8. Statistical Analysis
3. Results
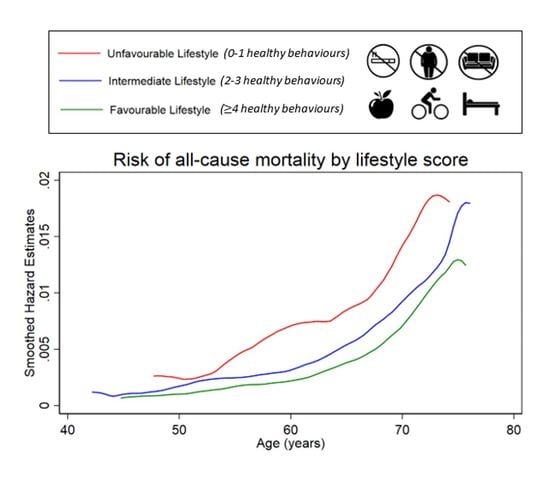
3.1. Unhealthy Lifestyle and Risk of All-Cause Mortality
3.2. Unhealthy Lifestyle and Risk of CVD Mortality
3.3. Unhealthy Lifestyle and Risk of Non-Fatal CVD Events
4. Discussion
4.1. Implications of This Research
4.2. Strengths and Limitations
4.3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation 2015, 132, 2212–2219. [Google Scholar] [CrossRef] [Green Version]
- Miller, V.; Webb, P.; Micha, R.; Mozaffarian, D. Defining diet quality: A synthesis of dietary quality metrics and their validity for the double burden of malnutrition. Lancet Planet. Health 2020, 4, e352–e370. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Chen, C.; Pan, X.F.; Guo, J.; Li, Y.; Franco, O.H.; Liu, G.; Pan, A. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: Two prospective cohort studies. BMJ 2021, 373, n604. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Ekelund, U.; Tarp, J.; Steene-Johannessen, J.; Hansen, B.H.; Jefferis, B.; Fagerland, M.W.; Whincup, P.; Diaz, K.M.; Hooker, S.P.; Chernofsky, A.; et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ 2019, 366, l4570. [Google Scholar] [CrossRef] [Green Version]
- St-Onge, M.-P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the american heart association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutten-Jacobs, L.C.; Larsson, S.C.; Malik, R.; Rannikmäe, K.; Sudlow, C.L.; Dichgans, M.; Markus, H.S.; Traylor, M. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: Cohort study of 306 473 UK Biobank participants. BMJ Br. Med. J. 2018, 363, k4168. [Google Scholar] [CrossRef] [Green Version]
- Said, M.A.; Verweij, N.; van der Harst, P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK biobank study. JAMA Cardiol. 2018, 3, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Nam, J.Y.; Kwon, S.; Keum, N.; Lee, J.-T.; Shin, M.-J.; Oh, H. Lifestyle risk score and mortality in Korean adults: A population-based cohort study. Sci. Rep. 2020, 10, 10260. [Google Scholar] [CrossRef]
- Khera, A.V.; Emdin, C.A.; Drake, I.; Natarajan, P.; Bick, A.G.; Cook, N.R.; Chasman, D.I.; Baber, U.; Mehran, R.; Rader, D.J.; et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N. Engl. J. Med. 2016, 375, 2349–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingstone, K.M.; McNaughton, S.A. A health behavior score is associated with hypertension and obesity among australian adults. Obesity 2017, 25, 1610–1617. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Rogers, K.; van der Ploeg, H.; Stamatakis, E.; Bauman, A.E. Traditional and emerging lifestyle risk behaviors and all-cause mortality in middle-aged and older adults: Evidence from a large population-based australian cohort. PLoS Med. 2015, 12, e1001917. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, nutrients, and dietary patterns: Interconnections and implications for dietary guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Kanauchi, M.; Kanauchi, K. The World Health Organization’s Healthy Diet indicator and its associated factors: A cross-sectional study in central Kinki, Japan. Prev. Med. Rep. 2018, 12, 198–202. [Google Scholar] [CrossRef]
- Voruganti, V.S. Nutritional genomics of cardiovascular disease. Curr. Genet. Med. Rep. 2018, 6, 98–106. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Abbott, G.; Bowe, S.J.; Ward, J.; Milte, C.; McNaughton, S.A. Diet quality indices, genetic risk and risk of cardiovascular disease and mortality: A longitudinal analysis of 77 004 UK Biobank participants. BMJ Open 2021, 11, e045362. [Google Scholar] [CrossRef]
- Jeannette, R.; Narita, A.; Manning, B.; McNaughton, S.A.; Mathers, J.C.; Livingstone, K.M. Does personalized nutrition advice improve dietary intake in healthy adults? A systematic review of randomized controlled trials. Adv. Nutr. 2021, 12, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, L.P.; Desikan, R.S. What are polygenic scores and why are they important? JAMA 2019, 321, 1820–1821. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, K.E.; Young, H.J.; Guo, W.; Key, T.J. Dietary assessment in UK Biobank: An evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 2018, 7, e6. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; Spencer, E.A.; Key, T.J.; Appleby, P.N.; Beral, V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011, 14, 1998–2005. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, D.C.; Hardie, L.J.; Frost, G.S.; Alwan, N.A.; Bradbury, K.E.; Carter, M.; Elliott, P.; Evans, C.E.L.; Ford, H.E.; Hancock, N.; et al. Validation of the oxford webq online 24-hour dietary questionnaire using biomarkers. Am. J. Epidemiol. 2019, 188, 1858–1867. [Google Scholar] [CrossRef] [Green Version]
- Food Standards Agency: Food Portion Sizes; The Stationary Office: London, UK, 2005.
- Royal Society of Chemistry; Ministry of Agriculture FaF. McCance and Widdowson’s the Composition of Foods, 6th ed.; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Waijers, P.M.C.M.; Feskens, E.J.M.; Ocké, M.C. A critical review of predefined diet quality scores. Br. J. Nutr. 2007, 97, 219–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Association of changes in diet quality with total and cause-specific mortality. N. Engl. J. Med. 2017, 377, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Schatzkin, A.; Graubard, B.I.; Schairer, C. A prospective study of diet quality and mortality in women. JAMA 2000, 283, 2109–2115. [Google Scholar] [CrossRef] [Green Version]
- Maynard, M.; Gunnell, D.; Ness, A.R.; Abraham, L.; Bates, C.J.; Blane, D. What influences diet in early old age? Prospective and cross-sectional analyses of the Boyd Orr cohort. Eur. J. Public Health 2005, 16, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Kant, A.K.; Graubard, B.I. A comparison of three dietary pattern indexes for predicting biomarkers of diet and disease. J. Am. Coll. Nutr. 2005, 24, 294–303. [Google Scholar] [CrossRef]
- Healthy Diet WHO Fact Sheet, No. 394. 2015. Available online: http://www.who.int/mediacentre/factsheets/fs394/en/ (accessed on 11 June 2021).
- BMI Classification. Available online: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed on 13 June 2021).
- IPAQ Scoring Protocol—International Physical Activity Questionnaire. Available online: https://sites.google.com/site/theipaq/scoring-protocol (accessed on 22 May 2020).
- Morris, J.S.; Bradbury, K.E.; Cross, A.J.; Gunter, M.J.; Murphy, N. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br. J. Cancer 2018, 118, 920–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, R.F.; Murray, J.M.; Coleman, H.G. The association between recreational screen time and cancer risk: Findings from the UK Biobank, a large prospective cohort study. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Sleep Needs across Lifespan. Available online: http://www.sleephealthfoundation.org.au/industry-professionals/about-sleep-health-foundation.html (accessed on 15 June 2021).
- Ntalla, I.; Kanoni, S.; Zeng, L.; Giannakopoulou, O.; Danesh, J.; Watkins, H.; Samani, N.J.; Deloukas, P.; Schunkert, H. Genetic risk score for coronary disease identifies predispositions to cardiovascular and noncardiovascular diseases. J. Am. Coll. Cardiol. 2019, 73, 2932–2942. [Google Scholar] [CrossRef]
- Magliano, D.; Liew, D.; Pater, H.; Kirby, A.; Hunt, D.; Simes, J.; Sundararajan, V.; Tonkin, A. Accuracy of the Australian national death index: Comparison with adjudicated fatal outcomes among Australian participants in the Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) study. Aust. N. Z. J. Public Health 2003, 27, 649–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnier, C.; Bush, K.; Nolan, J.C.S. Definitions of Stroke for UK Biobank Dhase 1 Outcomes Adjudication Documentation Prepared by: On Behalf of UK Biobank Outcome Adjudication Group. 2017. Available online: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/alg_outcome_stroke.pdf (accessed on 12 June 2021).
- Schnier, C.; Bush, K.; Nolan, J.C.S. Definitions of Acute Myocardial Infarction and Main Myocardial Infarction Pathological Types UK Biobank Phase 1 Outcomes Adjudication Documentation Prepared by: On Behalf of UK Biobank Outcome Adjudication Group. 2017. Available online: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/alg_outcome_mi.pdf (accessed on 15 June 2021).
- Towsend, P.; Phillimore, P.; Beattie, A. Health and deprivation. Nurs. Stand. 1988, 2, 34. [Google Scholar]
- Woodward, M. Rationale and tutorial for analysing and reporting sex differences in cardiovascular associations. Heart 2019, 105, 1701–1708. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.-S.; Jung, S.-H.; Shivakumar, M.; Xiao, B.; Khera, A.V.; Park, W.-Y.; Won, H.-H.; Kim, D. Polygenic risk, lifestyle, and cardiovascular mortality: A prospective population-based UK Biobank study. medRxiv 2021. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361, bmj.k2173. [Google Scholar] [CrossRef] [Green Version]
- Maruthur, N.M.; Wang, N.-Y.; Appel, L.J. Lifestyle interventions reduce coronary heart disease risk. Circulation 2009, 119, 2026–2031. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Overall N (%) | Males N (%) | Females N (%) |
|---|---|---|---|
| n | 76,958 | 34,968 (45.4) | 41,990 (54.6) |
| Age at recruitment (years), Mean ± SD | 56.2 ± 7.8 | 57.0 ± 7.8 | 55.6 ± 7.7 |
| Townsend Deprivation Index 1 | |||
| Least deprived | 18,115 (23.5) | 8605 (24.6) | 9510 (22.7) |
| 2nd least deprived | 17,222 (22.4) | 7910 (22.6) | 9312 (22.2) |
| Medium deprivation | 16,062 (20.9) | 7156 (20.5) | 8906 (21.2) |
| 2nd most deprived | 14,891 (19.4) | 6547 (18.7) | 8344 (19.9) |
| Most deprived | 10,668 (13.9) | 4750 (13.6) | 5918 (14.1) |
| Body Mass Index (kg/m2), Mean ± SD | 26.5 ± 4.4 | 27.1 ± 3.9 | 26.0 ± 4.7 |
| Waist circumference (cm), Mean ± SD | 88.1 ± 13.0 | 95.2 ± 10.8 | 82.3 ± 11.6 |
| Total PA (MET min), Mean ± SD | 2477 ± 2326 | 2542 ± 2439 | 2423 ± 2227 |
| Medication use 2 | 16,562 (21.5) | 9707 (27.8) | 6855 (16.3) |
| Family history of CVD | 57,182 (74.3) | 25,068 (71.7) | 32,114 (76.5) |
| Favourable lifestyle behaviours 3 | |||
| Non-smoker | 71,995 (93.6) | 32,305 (92.4) | 39,690 (94.5) |
| No overweight/obesity | 30,173 (39.2) | 10,543 (30.2) | 19,630 (46.8) |
| Physically active | 23,131 (30.1) | 10,589 (30.3) | 12,542 (29.9) |
| Healthy diet | 41,877 (54.4) | 18,495 (52.9) | 23,382 (55.7) |
| Not sedentary | 73,305 (95.3) | 32,821 (93.9) | 40,484 (96.4) |
| Optimal sleep | 60,545 (78.7) | 27,364 (78.3) | 33,181 (79.0) |
| Lifestyle score | |||
| 4 or more favourable lifestyle behaviours | 43,118 (56.0) | 17,516 (50.1) | 25,602 (61.0) |
| 2 or 3 favourable lifestyle behaviours | 31,363 (40.8) | 16,019 (45.8) | 15,344 (36.5) |
| 0 or 1 favourable lifestyle behaviours | 2477 (3.2) | 1433 (4.1) | 1044 (2.49) |
| Overall (n = 76,958) | Males (n = 34,968) | Females (n = 41,990) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | HR | 95% CI | p-Value | Cases | HR | 95% CI | p-Value | Cases | HR | 95% CI | p-Value | |
| All-cause mortality | 2408 | 1415 | 993 | |||||||||
| Favourable LS | 1120 | 1.00 | 594 | 1.00 | 526 | 1.00 | ||||||
| Intermediate LS | 1146 | 1.31 | 1.21, 1.43 | <0.001 | 721 | 1.33 | 1.20, 1.49 | <0.001 | 425 | 1.29 | 1.14, 1.47 | <0.001 |
| Unfavourable LS | 142 | 2.06 | 1.73, 2.45 | <0.001 | 100 | 2.17 | 1.76, 2.69 | <0.001 | 42 | 1.85 | 1.35, 2.54 | <0.001 |
| Polygenic risk score | 1.00 | 0.97, 1.05 | 0.64 | 1.02 | 0.97, 1.08 | 0.64 | 1.00 | 0.93, 1.05 | 0.74 | |||
| CVD mortality | 364 | 263 | 101 | |||||||||
| Favourable LS | 161 | 1.00 | 108 | 1.00 | 53 | 1.00 | ||||||
| Intermediate LS | 177 | 1.35 | 1.09, 1.67 | 0.007 | 133 | 1.35 | 1.05, 1.75 | 0.020 | 44 | 1.32 | 0.88, 1.96 | 0.18 |
| Unfavourable LS | 26 | 2.48 | 1.64, 3.76 | <0.001 | 22 | 2.66 | 1.68, 4.21 | <0.001 | 4 | 1.79 | 0.65, 4.95 | 0.26 |
| Polygenic risk score | 1.11 | 1.00, 1.23 | 0.05 | 1.13 | 1.00, 1.28 | 0.045 | 1.04 | 0.86, 1.27 | 0.68 | |||
| Myocardial Infarction | 1140 | 822 | 318 | |||||||||
| Favourable LS | 509 | 1.00 | 355 | 1.00 | 154 | 1.00 | ||||||
| Intermediate LS | 560 | 1.34 | 1.19, 1.52 | <0.001 | 410 | 1.27 | 1.10, 1.46 | 0.001 | 150 | 1.54 | 1.23, 1.92 | <0.001 |
| Unfavourable LS | 71 | 2.12 | 1.65, 2.72 | <0.001 | 57 | 2.11 | 1.60, 2.80 | <0.001 | 14 | 2.00 | 1.16, 3.47 | 0.013 |
| Polygenic risk score | 1.35 | 1.27, 1.43 | <0.001 | 1.42 | 1.33, 1.52 | <0.001 | 1.19 | 1.06, 1.32 | 0.003 | |||
| Stroke | 748 | 447 | 301 | |||||||||
| Favourable LS | 374 | 1.00 | 222 | 1.00 | 152 | 1.00 | ||||||
| Intermediate LS | 335 | 1.15 | 1.00, 1.34 | 0.059 | 201 | 1.00 | 0.83, 1.21 | 0.99 | 134 | 1.42 | 1.13, 1.79 | 0.003 |
| Unfavourable HLS | 39 | 1.74 | 1.25, 2.43 | 0.001 | 24 | 1.43 | 0.94, 2.18 | 0.10 | 15 | 2.37 | 1.39, 4.03 | 0.002 |
| Polygenic risk score | 1.02 | 0.95, 1.10 | 0.52 | 0.99 | 0.90, 1.08 | 0.75 | 1.08 | 0.97, 1.21 | 0.18 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livingstone, K.M.; Abbott, G.; Ward, J.; Bowe, S.J. Unhealthy Lifestyle, Genetics and Risk of Cardiovascular Disease and Mortality in 76,958 Individuals from the UK Biobank Cohort Study. Nutrients 2021, 13, 4283. https://doi.org/10.3390/nu13124283
Livingstone KM, Abbott G, Ward J, Bowe SJ. Unhealthy Lifestyle, Genetics and Risk of Cardiovascular Disease and Mortality in 76,958 Individuals from the UK Biobank Cohort Study. Nutrients. 2021; 13(12):4283. https://doi.org/10.3390/nu13124283
Chicago/Turabian StyleLivingstone, Katherine M., Gavin Abbott, Joey Ward, and Steven J. Bowe. 2021. "Unhealthy Lifestyle, Genetics and Risk of Cardiovascular Disease and Mortality in 76,958 Individuals from the UK Biobank Cohort Study" Nutrients 13, no. 12: 4283. https://doi.org/10.3390/nu13124283
APA StyleLivingstone, K. M., Abbott, G., Ward, J., & Bowe, S. J. (2021). Unhealthy Lifestyle, Genetics and Risk of Cardiovascular Disease and Mortality in 76,958 Individuals from the UK Biobank Cohort Study. Nutrients, 13(12), 4283. https://doi.org/10.3390/nu13124283