Bacillus toyonensis SAU-19 Ameliorates Hepatic Insulin Resistance in High-Fat Diet/Streptozocin-Induced Diabetic Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Preparation of Probiotic Bacteria Suspensions
2.3. Experimental Animal and Design
2.4. Oral Glucose Tolerance Test
2.5. Blood and Tissue Sample Collection
2.6. Biochemical Parameters
2.7. Liver Histological Analysis
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Statistical Analysis
3. Results
3.1. Effects of B. toyonensis Strain SAU-19 on Growth Performance in HFD/STZ-Induced T2DM Mice
3.2. Effects of B. toyonensis Strain SAU-19 on Blood Glucose Levels and Oral Glucose Tolerance Test (OGTT) in HFD/STZ-Induced T2DM Mice
3.3. Effects of B. toyonensis Strain SAU-19 on Biochemical Parameters in HFD/STZ-Induced T2DM Mice
3.4. Effects of B. toyonensis Strain SAU-19 on Lipid Profiles in HFD/STZ-Induced T2DM Mice
3.5. Effects of B. toyonensis Strain SAU-19 on Antioxidant Activity in HFD/STZ-Induced T2DM Mice
3.6. Effects of B. toyonensis Strain SAU-19 on Liver Histological Injury in HFD/STZ-Induced T2DM Mice
3.7. Effects of B. toyonensis Strain SAU-19 on Relative mRNA and Protein (ELISA) Expression of Genes Related to Inflammation in Liver Tissues of HFD/STZ-Induced T2DM Mice
3.8. Effects of B. toyonensis Strain SAU-19 on Relative mRNA Expression of Genes Related to Glucose and Glycogen Synthesis in the Liver Tissues of HFD/STZ-Induced T2DM Mice
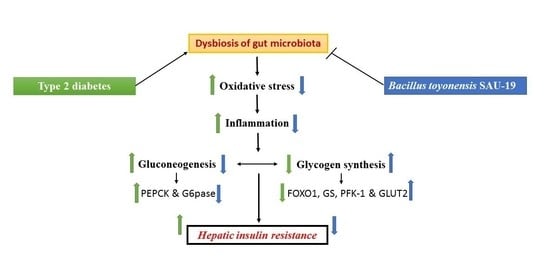
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S. Ameliorative Effects of Probiotic Lactobacillus paracasei NL41 on Insulin Sensitivity, Oxidative Stress, and Beta-Cell Function in a Type 2 Diabetes Mellitus Rat Model. Mol. Nutr. Food Res. 2019, 63, e1900457. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway. Br. J. Pharmacol. 2018, 175, 4218–4228. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates for the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Sun, H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 2018, 130, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Jimenez, G.; Blanch, A.R.; Tamames, J.; Rossello-Mora, R. Complete genome sequence of Bacillus toyonensis BCT-7112T, the active ingredient of the feed additive preparation Toyocerin. Genome Announc. 2013, 1, 01080-13. [Google Scholar] [CrossRef] [Green Version]
- EFSA. FEEDAP panel (EFSA panel on additives and products or substances used in animal feed) scientific opinion on the safety and efficacy of Toyocerin (Bacillus toyonensis) as a feed additive for rabbits for fattening, chickens for fattening, weaned piglets, pigs for fattening, sows for reproduction, cattle for fattening, and calve s for rearing. EFSA J. 2014, 12, 17. [Google Scholar]
- Taras, D.; Vahjen, W.; Macha, M.; Simon, O. Response of performance characteristics and fecal consistency to long-lasting dietary supplementation with the probiotic strain Bacillus cereus var. toyoi to sows and piglets. Arch. Anim. Nutr. 2005, 59, 405–417. [Google Scholar] [CrossRef]
- Baum, B.; Liebler-Tenorio, E.M.; Enss, M.L.; Pohlenz, J.F.; Breves, G. Saccharomyces boulardii and Bacillus cereus var. toyoi influence the morphology and the mucins of the intestine of pigs. Z. Gastroenterol. 2002, 40, 277–284. [Google Scholar] [CrossRef]
- Simon, O. An interdisciplinary study on the mode of action of probiotics in pigs. J. Anim. Feed Sci. 2010, 19, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Okyere, S.K.; Mo, Q.; Pei, G.; Ren, Z.; Deng, J.; Hu, Y. Euptox A Induces G0 /GI arrest and apoptosis of hepatocyte via ROS, mitochondrial dysfunction and caspases-dependent pathways in vivo. J. Toxicol. Sci. 2020, 45, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Okyere, S.K.; Wen, J.; Cui, Y.; Xie, L.; Gao, P.; Wang, J.; Wang, S.; Hu, Y. Toxic mechanisms and pharmacological properties of euptox A, a toxic monomer from A. adenophora. Fitoterapia 2021, 155, 105032. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Okyere, S.K.; Wen, J.; Xie, L.; Cui, Y.; Wang, S.; Wang, J.; Cao, S.; Shen, L.; Ma, X.; et al. An Overview: The Toxicity of Ageratina adenophora on Animals and Its Possible Interventions. Int. J. Mol. Sci. 2021, 22, 11581. [Google Scholar] [CrossRef]
- Nath, S.; Ghosh, S.K.; Choudhury, Y. A murine model of type 2 diabetes mellitus developed using a combination of high fat diet and multiple low doses of streptozotocin treatment mimics the metabolic characteristics of type 2 diabetes mellitus in humans. J. Pharmacol. Toxicol. Methods 2017, 84, 20–30. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, M.G.; Jiang, C.H.; Sheng, X.P.; Yang, H.M.; Liu, Y.; Yao, X.M.; Zhang, J.; Yin, Z.Q. Triterpenic acids-enriched fraction from Cyclocarya paliurus attenuates insulin resistance and hepatic steatosis via PI3K/Akt/GSK3β pathway. Phytomedicine 2020, 66, 153130. [Google Scholar] [CrossRef]
- Chen, R.; Ping, Z.; Chen, J.; Liang, L.; Lu, C. Mycelia Extract from Spent Ganoderma Lucidum Bagasse Substrate Ameliorates Carbon Tetrachloride-Induced Acute Liver Injury in Mice Through Inhibiting Oxidative Stress. Waste Biomass Valor 2021, 12, 3257–3269. [Google Scholar] [CrossRef]
- Cui, Y.; Okyere, S.K.; Gao, P.; Wen, J.; Cao, S.; Wang, Y.; Deng, J.; Hu, Y. Ageratina adenophora Disrupts the Intestinal Structure and Immune Barrier Integrity in Rats. Toxins 2021, 13, 651. [Google Scholar] [CrossRef]
- Li, S.; Huang, Q.; Zhang, L.; Qiao, X.; Zhang, Y.; Tang, F.; Li, Z. Effect of CAPE-pNO2 against type 2 diabetes mellitus via the AMPK/GLUT4/ GSK3β/PPARα pathway in HFD/STZ-induced diabetic mice. Eur. J. Pharmacol. 2019, 853, 1–10. [Google Scholar] [CrossRef]
- Kantas, D.; Papatsiros, V.G.; Tassis, P.D.; Giavasis, I.; Bouki, P.; Tzika, E.D. A feed additive containing Bacillus toyonensis (Toyocerin (®)) protects against enteric pathogens in postweaning piglets. J. Appl. Microbiol. 2015, 118, 727–738. [Google Scholar] [CrossRef]
- Zafar, M.; Naeem-Ul-Hassan Naqvi, S. Effects of STZ-Induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: A comparative study. Int. J. Morphol. 2010, 28, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Shu, G.; Kong, F.; Xu, D.; Yin, L.; He, C.; Lin, J.; Fu, H.; Wang, K.; Tian, Y.; Zhao, X. Bamboo leaf flavone changed the community of cecum microbiota and improved the immune function in broilers. Sci. Rep. 2020, 10, 12324. [Google Scholar] [CrossRef]
- Iftikhar, H.; Mahmood, M.S.; Arshad, M.I.; Akhtar, M.; Mahmood, F.; Rafique, A. Immune system dysfunction in broiler chickens experimentally inoculated with fowl adenovirus serotype-4 associated with inclusion body hepatitis hydropericardium syndrome. Turk. J. Vet. Anim. Sci. 2012, 36, 223–230. [Google Scholar]
- da Costa, W.K.A.; Brandão, L.R.; Martino, M.E.; Garcia, E.F.; Alves, A.F.; de Souza, E.L.; de Souza Aquino, J.; Saarela, M.; Leulier, F.; Vidal, H.; et al. Qualification of tropical fruit-derived Lactobacillus plantarum strains as potential probiotics acting on blood glucose and total cholesterol levels in Wistar rats. Food Res. Int. 2019, 124, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Farida, E.; Nuraida, L.; Giriwono, P.E.; Jenie, B.S.L. Lactobacillus rhamnosus Reduces Blood Glucose Level through Downregulation of Gluconeogenesis Gene Expression in Streptozotocin-Induced Diabetic Rats. Int. J. Food Sci. 2020, 2020, 6108575. [Google Scholar] [CrossRef] [Green Version]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, L.; Shive, C.L.; Zebrowski, E.; Fuller, B.; Rife, K.; Hirsch, A.; Compan, A.; Moreland, A.; Falck-Ytter, Y.; Popkin, D.L.; et al. Soluble Markers of Immune Activation Differentially Normalize and Selectively Associate with Improvement in AST, ALT, Albumin, and Transient Elastography During IFN-Free HCV Therapy. Pathog. Immun. 2018, 3, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Choudhary, N.S.; Singh, M.K.; Wasir, J.S.; Kaur, P.; Gill, H.K.; Bano, T.; Farooqui, K.J.; et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: Randomised controlled trial (D-LIFT trial). Diabetologia 2020, 63, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, L.; Zhang, G.; Varkaneh, H.K.; Rahmani, J.; Clark, C.; Ryan, P.M.; Abdulazeem, H.M.; Salehisahlabadi, A. The association of plasma levels of liver enzymes and risk of gestational diabetes mellitus: A systematic review and dose-response meta-analysis of observational studies. Acta Diabetol. 2020, 57, 635–644. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Huseini, H.F.; Derakhshanian, H.; Khodaii, Z.; Tavakoli-Far, B. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J. Diabetes Metab. Disord. 2020, 19, 53–60. [Google Scholar] [CrossRef]
- Albuquerque, R.C.M.F.; Brandão, A.B.P.; De Abreu, I.C.M.E.; Ferreira, F.G.; Santos, L.B.; Moreira, L.N.; Taddei, C.R.; Aimbire, F.; Cunha, T.S. Saccharomyces boulardii Tht 500101 changes gut microbiota and ameliorates hyperglycaemia, dyslipidaemia, and liver inflammation in streptozotocin-diabetic mice. Benef. Microbes 2019, 10, 901–912. [Google Scholar] [CrossRef]
- Sampath, K.A.; Maiya, A.G.; Shastry, B.A.; Vaishali, K.; Ravishankar, N.; Hazari, A.; Gundmi, S.; Jadhav, R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.S.; Zhu, C.J.; Yan, B.Y.; Yan, C.Y.; Ling, R. Stimulation of KLF14/PLK1 pathway by thrombin signaling potentiates endothelial dysfunction in Type 2 diabetes mellitus. Biomed. Pharmacother. 2018, 99, 859–866. [Google Scholar] [CrossRef]
- Zhou, S.; Dai, Y.M.; Zeng, X.F.; Chen, H.Z. Circadian Clock and Sirtuins in Diabetic Lung: A Mechanistic Perspective. Front. Endocrinol. 2020, 11, 173. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia 2016, 59, 679–682. [Google Scholar] [CrossRef]
- Elimam, H.; Abdulla, A.M.; Taha, I.M. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 800–804. [Google Scholar] [CrossRef]
- Herder, C.; Carstensen, M.; Ouwens, D.M. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 39–50. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Li, F.; Gu, Z.; Liu, M.; Shao, T.; Zhang, L.; Zhou, G.; Pan, C.; He, L.; et al. Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice. J. Nutr. Biochem. 2020, 75, 108256. [Google Scholar] [CrossRef]
- Cui, X.; Qian, D.W.; Jiang, S.; Shang, E.X.; Zhu, Z.H.; Duan, J.A. Scutellariae Radix and Coptidis Rhizoma Improve Glucose and Lipid Metabolism in T2DM Rats via Regulation of the Metabolic Profiling and MAPK/PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lv, Q.; Jia, S.; Chen, Y. Effects of flavonoid-rich Chinese bayberry (Morella rubra Sieb. et Zucc.) fruit extract on regulating glucose and lipid metabolism in diabetic KKA(y) mice. Food Funct. 2016, 7, 3130–3140. [Google Scholar] [CrossRef]
- Shi, C.X.; Zhao, M.X.; Shu, X.D.; Xiong, X.Q.; Wang, J.J.; Gao, X.Y.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Beta-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci. Rep. 2016, 6, 21924. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, F.G.; Song, W.; Yang, Y.L.; Liu, J.F.; Li, P.; Liu, B.L.; Qi, W. Ginsenoside Rg1 inhibits glucagon-induced hepatic gluconeogenesis through Akt-FoxO1 interaction. Arch. Med. Res. 2018, 49, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Shin, H.M.; Jung, H.; Rhee, B.D.; Park, J.H. Comparison of pancreatic beta cells and alpha cells under hyperglycemia: Inverse coupling in pAkt-FoxO1. Diabetes Res. Clin. Pract. 2017, 131, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kolnes, A.J.; Birk, J.B.; Eilertsen, E.; Stuenaes, J.T.; Wojtaszewski, J.F.; Jensen, J. Epinephrine-stimulated glycogen breakdown activates glycogen synthase and increases insulin-stimulated glucose uptake in epitrochlearis muscles. Am. J. Physiol. Endocrinol. 2015, 308, E231–E240. [Google Scholar] [CrossRef] [Green Version]
- Hussar, P.; Karner, M.; Jarveots, T.; Pendovski, L.; Duritis, I.; Popovska-Percinic, F. Comparative study of glucose transporters GLUT-2 and GLUT-5 in ostriches gastrointestinal tract. Mac. Vet. Rev. 2016, 39, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; Tomita, S.; Sekiya, M.; et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes 2004, 53, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Kang, M.A.; Choi, M.S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006, 38, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Tanaka, S.; Haga, Y. Enhanced GLUT2 gene expression in anoleic acid-induced in vitro fatty liver model. Hepatol, Res. 2002, 23, 138–144. [Google Scholar] [CrossRef]
- Mohammed, K.A.A.; Ahmed, H.M.S.; Sharaf, H.A.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Mehaya, F.M.; Abdel-Wahhab, M.A. Encapsulation of cinnamon oil in whey protein counteracts the disturbances in biochemical parameters, gene expression, and histological picture of the liver and pancreas of diabetic rats. Environ. Sci. Pollut. Res. 2020, 27, 2829–2843. [Google Scholar] [CrossRef]
- Song, J.; Gao, J.; Du, M.; Mao, X.Y. Casein lycomacropeptide hydrolysates ameliorate hepatic insulin resistance of C57BL/6J mice challenged with high-fat diet. J. Funct. 2018, 45, 190–198. [Google Scholar] [CrossRef]
- Lang, L.; Chemmalakuzhy, R.; Shay, C.; Teng, Y. PFKP Signaling at a Glance: An Emerging Mediator of Cancer Cell Metabolism. Adv. Exp. Med. Biol. 2019, 1134, 243–258. [Google Scholar]
- Bockus, L.B.; Matsuzaki, S.; Vadvalkar, S.S.; Young, Z.T.; Giorgione, J.R.; Newhardt, M.F.; Kinter, M.; Humphries, K.M. Cardiac Insulin Signaling Regulates Glycolysis Through Phosphofructokinase 2 Content and Activity. J. Am. Heart Assoc. 2017, 6, e007159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, R.; Dey, D.K.; Vij, R.; Meena, S.; Kapila, R.; Kapila, S. Evaluation of anti-diabetic attributes of Lactobacillus rhamnosus MTCC: 5957, Lactobacillus rhamnosus MTCC: 5897 and Lactobacillus fermentum MTCC: 5898 in streptozotocin induced diabetic rats. Microb. Pathog. 2018, 125, 454–462. [Google Scholar] [CrossRef]
- Kim, S.H.; Huh, C.S.; Choi, I.D.; Jeong, J.W.; Ku, H.K.; Ra, J.H.; Kim, T.Y.; Kim, G.B.; Sim, J.H.; Ahn, Y.T. The anti-diabetic activity of Bifidobacterium lactis HY8101 in vitro and in vivo. J. Appl. Microbiol. 2014, 117, 834–845. [Google Scholar] [CrossRef]









| Normal Diet | High-Fat Diet | ||
|---|---|---|---|
| Ingredients | Content g/kg | Ingredients | Content g/kg |
| Water | 94 | Water | 93 |
| Protein | 190 | Protein | 134 |
| Fat | 51 | Fat | 143 |
| Fiber | 36 | Fiber | 27 |
| Ash | 62 | Ash | 44 |
| Calcium | 11.3 | Calcium | 8.3 |
| Phosphorus | 8.6 | Phosphorus | 7.1 |
| Gene Name | Primer | Sequence (5′ and 3′) | Product Length (bp) | Annealing Temperature (°C) | Sequence Number |
|---|---|---|---|---|---|
| IL-1β | Forward | TGAAATGCCACCTTTGACAGTG | 141 | 60.18 | NM_008361.4 |
| Reverse | ATGTGCTGCTGCGAGATTTG | ||||
| PEPCK | Forward | GACAGACTCGCCCTATGTGG | 98 | 59.90 | NM_011044.3 |
| Reverse | GGCACTTGATGAACTCCCCA | ||||
| IL-4 | Forward | GTACCAGGAGCCATATCCACG | 130 | 60.18 | NM_021283.2 |
| Reverse | TTCGTTGCTGTGAGGACGTT | ||||
| IL-10 | Forward | GGGGCGAGTGTAACAAGACC | 109 | 60.27 | XM_036162094.1 |
| Reverse | GCAGAGGAGGTCACACCATTT | ||||
| TNF-α | Forward | CCCTCACACTCACAAACCAC | 211 | 59.82 | NM_001278601.1 |
| Reverse | ATAGCAAATCGGCTGACGGT | ||||
| g6pc | Forward | GTTTGGTTTCGCGCTTGGAT | 95 | 59.82 | NM_008061.4 |
| Reverse | GCCGCTCACACCATCTCTTA | ||||
| FoxO1 | Forward | AGTGGATGGTGAAGAGCGTG | 96 | 60.04 | NM_019739.3 |
| Reverse | GAAGGGACAGATTGTGGCGA | ||||
| GS | Forward | AGGATGAATTCGACCCCGAG | 81 | 55.00 | NM_030678.3 |
| Reverse | CAGTGTAGATGCCACCCACC | ||||
| Glut2 | Forward | GATCACCGGAACCTTGGCTT | 76 | 55.00 | NM_031197.2 |
| Reverse | CACACCGATGTCATAGCCGA | ||||
| Pfkl | Forward | AAAGCGGCGTGTGTTCATTG | 73 | 60.39 | NM_008826.5 |
| Reverse | AGCAATGCCGGTCACAGTAG | ||||
| β-actin | Forward | TTCGCGGGCGACGAT | 297 | 58.57 | NM_0077393.5 |
| Reverse | CATCTTTTCACGGTTGGCCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okyere, S.K.; Xie, L.; Wen, J.; Ran, Y.; Ren, Z.; Deng, J.; Hu, Y. Bacillus toyonensis SAU-19 Ameliorates Hepatic Insulin Resistance in High-Fat Diet/Streptozocin-Induced Diabetic Mice. Nutrients 2021, 13, 4512. https://doi.org/10.3390/nu13124512
Okyere SK, Xie L, Wen J, Ran Y, Ren Z, Deng J, Hu Y. Bacillus toyonensis SAU-19 Ameliorates Hepatic Insulin Resistance in High-Fat Diet/Streptozocin-Induced Diabetic Mice. Nutrients. 2021; 13(12):4512. https://doi.org/10.3390/nu13124512
Chicago/Turabian StyleOkyere, Samuel Kumi, Lei Xie, Juan Wen, Yinan Ran, Zhihua Ren, Junliang Deng, and Yanchun Hu. 2021. "Bacillus toyonensis SAU-19 Ameliorates Hepatic Insulin Resistance in High-Fat Diet/Streptozocin-Induced Diabetic Mice" Nutrients 13, no. 12: 4512. https://doi.org/10.3390/nu13124512
APA StyleOkyere, S. K., Xie, L., Wen, J., Ran, Y., Ren, Z., Deng, J., & Hu, Y. (2021). Bacillus toyonensis SAU-19 Ameliorates Hepatic Insulin Resistance in High-Fat Diet/Streptozocin-Induced Diabetic Mice. Nutrients, 13(12), 4512. https://doi.org/10.3390/nu13124512