Analytical Performance Specifications for 25-Hydroxyvitamin D Examinations
Abstract
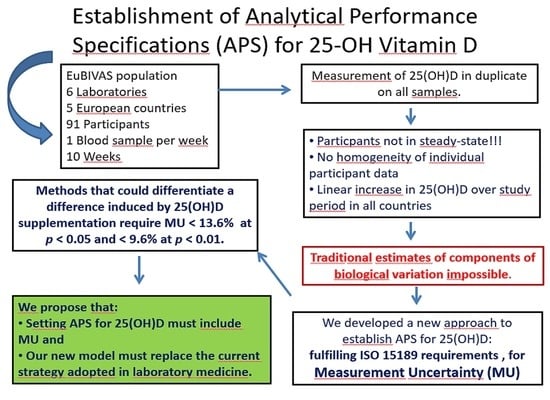
:1. Introduction
2. Materials and Methods
2.1. Biological Variation Data Derived from the EuBIVAS
2.2. Data Analysis
2.3. Analytical Methods
3. Results
3.1. APS for 25(OH)D Based upon Biological Variation Derived from the EuBIVAS, EFLM Model 2
3.2. APS for 25(OH)D Based on the Effect of MU on Change in Serial Results, EFLM Model 1
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
Disclaimer
References
- Hollis, B.W. The determination of circulating 25-hydroxyvitamin D: No easy task. J. Clin. Endocrinol. Metab. 2004, 89, 3149–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalier, E.; Souberbielle, J.-C. Vitamin D and its metabolites: From now and beyond. EJIFCC 2018, 29, 105–110. [Google Scholar] [PubMed]
- Sempos, C.T.; Betz, J.M.; Camara, J.E.; Carter, G.D.; Cavalier, E.; Clarke, M.W.; Dowling, K.G.; Durazo-Arvizu, R.A.; Hoofnagle, A.N.; Liu, A.; et al. General steps to standardize the laboratory measurement of serum total 25-hydroxyvitamin D. J. AOAC Int. 2017, 100, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.S.-C.; Bedner, M.; Phinney, K.W. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal. Chem. 2010, 82, 1942–1948. [Google Scholar] [CrossRef] [Green Version]
- Stepman, H.C.M.; Vanderroost, A.; Van Uytfanghe, K.; Thienpont, L.M. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-Hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin. Chem. 2011, 57, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Mineva, E.M.; Schleicher, R.L.; Chaudhary-Webb, M.; Maw, K.L.; Botelho, J.C.; Vesper, H.W.; Pfeiffer, C.M. A candidate reference measurement procedure for quantifying serum concentrations of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 407, 5615–5624. [Google Scholar] [CrossRef]
- Stöckl, D.; Sluss, P.M.; Thienpont, L.M. Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin. Chim. Acta 2009, 408, 8–13. [Google Scholar] [CrossRef]
- CDC Vitamin D Standardization-Certification Program (CDC VDSCP) Certified Total 25-Hydroxyvitamin D Procedures. Available online: https://www.cdc.gov/labstandards/pdf/hs/CDC_Certified_Vitamin_D_Procedures-508.pdf (accessed on 25 November 2020).
- Fraser, C.G. The 1999 Stockholm Consensus Conference on quality specifications in laboratory medicine. Clin. Chem. Lab. Med. 2015, 53, 837–840. [Google Scholar] [CrossRef]
- Sandberg, S.; Fraser, C.G.; Horvath, A.R.; Jansen, R.; Jones, G.; Oosterhuis, W.; Petersen, P.H.; Schimmel, H.; Sikaris, K.; Panteghini, M. Defining analytical performance specifications: Consensus statement from the 1st strategic conference of the European Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. Lab. Med. 2015, 53, 833–835. [Google Scholar] [CrossRef] [Green Version]
- Aarsand, A.K.; Fernandez-Calle, P.; Webster, C.; Coskun, A.; Gonzales-Lao, E.; Diaz-Garzón, J.; Jonker, N.; Minchinela, J.; Simon, M.; Braga, F.; et al. The EFLM Biological Variation Database; European Federation of Clinical Chemistry and Laboratory Medicine: Milano, Italy. Available online: https://biologicalvariation.eu/ (accessed on 25 November 2020).
- Lutsey, P.L.; Parrinello, C.M.; Misialek, J.R.; Hoofnagle, A.N.; Henderson, C.M.; Laha, T.J.; Michos, E.D.; Eckfeldt, J.H.; Selvin, E. Short-Term variability of Vitamin D-related biomarkers. Clin. Chem. 2016, 62, 1647–1653. [Google Scholar] [CrossRef] [Green Version]
- Lacher, D.A.; Hughes, J.P.; Carroll, M.D. Biological variation of laboratory analytes based on the 1999–2002 National Health and Nutrition Examination Survey. Natl. Health Stat. Rep. 2010, 1, 1–7. [Google Scholar]
- Viljoen, A.; Singh, D.K.; Farrington, K.; Twomey, P.J. Analytical quality goals for 25-vitamin D based on biological variation. J. Clin. Lab. Anal. 2011, 25, 130–133. [Google Scholar] [CrossRef]
- Ceriotti, F.; Fernandez-Calle, P.; Klee, G.G.; Nordin, G.; Sandberg, S.; Streichert, T.; Vives-Corrons, J.L.; Panteghini, M. Criteria for assigning laboratory measurands to models for analytical performance specifications defined in the 1st EFLM Strategic Conference. Clin. Chem. Lab. Med. 2017, 55, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrance, I.; Badrick, T.; Frenkel, R. Uncertainty in measurement and total error: Different roads to the same quality destination? Clin. Chem. Lab. Med. 2018, 56, 2010–2014. [Google Scholar] [CrossRef] [PubMed]
- Carobene, A.; Strollo, M.; Jonker, N.; Barla, G.; Bartlett, W.A.; Sandberg, S.; Sylte, M.S.; Røraas, T.; Sølvik, U.Ø.; Fernandez-Calle, P.; et al. Sample collections from healthy volunteers for biological variation estimates’ update: A new project undertaken by the working group on biological variation established by the European Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. Lab. Med. 2016, 54, 1599–1608. [Google Scholar] [CrossRef]
- Aarsand, A.K.; Røraas, T.; Bartlett, W.A.; Coşkun, A.; Carobene, A.; Fernandez-Calle, P.; Jonker, N.; Díaz-Garzón, J.; Braga, F.; Sandberg, S. Harmonization initiatives in the generation, reporting and application of biological variation data. Clin. Chem. Lab. Med. 2018, 56, 1629–1636. [Google Scholar] [CrossRef] [Green Version]
- Røraas, T.; Petersen, P.H.; Sandberg, S. Confidence intervals and power calculations for within-person biological variation: Effect of analytical imprecision, number of replicates, number of samples, and number of individuals. Clin. Chem. 2012, 58, 1306–1313. [Google Scholar]
- Wielders, J.P.M.; Wijnberg, F.A. Preanalytical stability of 25(OH)-vitamin D3 in human blood or serum at room temperature: Solid as a rock. Clin. Chem. 2009, 55, 1584–1585. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.G.; Elder, P.A. Serum 25-OH Vitamin D2 and D3 are stable under exaggerated conditions. Clin. Chem. 2008, 54, 1931–1932. [Google Scholar] [CrossRef] [Green Version]
- Carobene, A.; Guerra, E.; Locatelli, M.; Cucchiara, V.; Briganti, A.; Aarsand, A.K.; Coşkun, A.; Díaz-Garzón, J.; Fernandez-Calle, P.; Røraas, T.; et al. Biological variation estimates for prostate specific antigen from the European Biological Variation Study; consequences for diagnosis and monitoring of prostate cancer. Clin. Chim. Acta 2018, 486, 185–191. [Google Scholar] [CrossRef]
- Carobene, A.; Aarsand, A.K.; Guerra, E.; Bartlett, W.A.; Coşkun, A.; Díaz-Garzón, J.; Fernandez-Calle, P.; Jonker, N.; Locatelli, M.; Sandberg, S.; et al. European biological variation study (EUBIVAS): Within-And between-subject biological variation data for 15 frequently measured proteins. Clin. Chem. 2019, 65, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, E.; Lukas, P.; Bekaert, A.-C.; Peeters, S.; Le Goff, C.; Yayo, E.; Delanaye, P.; Souberbielle, J.-C. Analytical and clinical evaluation of the new Fujirebio Lumipulse®G non-competitive assay for 25(OH)-vitamin D and three immunoassays for 25(OH)D in healthy subjects, osteoporotic patients, third trimester pregnant women, healthy African subjects, hemodialyzed patients. Clin. Chem. Lab. Med. 2015, 1–9. [Google Scholar] [CrossRef]
- Aarsand, A.K.; Diaz-Garzon, J.; Fernandez-Calle, P.; Guerra, E.; Locatelli, M.; Bartlett, W.A.; Sandberg, S.; Roraas, T.; Ceriotti, F.; Sølvik, U.Ø.; et al. The EuBIVAS: Within-And between-subject biological variation data for electrolytes, lipids, urea, uric acid, total protein, total bilirubin, direct bilirubin, and glucose. Clin. Chem. 2018, 64, 1380–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochran, W.G. The distribution of the largest of a set of estimated variances as a fraction of their total. Ann. Hum. Genet. 1941, 11, 47–52. [Google Scholar] [CrossRef]
- Souberbielle, J.-C.; Body, J.-J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P.; et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010, 9, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Stamp, T.C.B.; Round, J.M. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature 1974, 247, 563–565. [Google Scholar] [CrossRef]
- ISO—ISO 15189:2012—Medical Laboratories—Requirements for Quality and Competence. Available online: https://www.iso.org/standard/56115.html (accessed on 7 July 2020).
- Joint Committee for Guides in Metrology (JCGM). International Vocabulary of Metrology-Basic and General Concepts and Associated Terms (VIM), 3rd ed.; Joint Committee for Guides in Metrology (JCGM): Paris, France, 2008. [Google Scholar]
- Brunel, E.; Schnitzler, M.; Foidart-Dessalle, M.; Souberbielle, J.C.; Cavalier, E. A double-blind, placebo controlled, randomized trial to assess the impact of a monthly administration of 50,000 IU of vitamin D3 for 6 months on serum levels of 25-hydroxyvitamin d in healthy young adults. Int. J. Endocrinol. 2013, 2013. [Google Scholar] [CrossRef]
- Stepman, H.C.M.; Thienpont, L.M. Measurement uncertainty for the analysis of serum 25-hydroxyvitamin D. Osteoporos. Int. 2010, 21, 1053. [Google Scholar]
- Cavalier, E.; Rozet, E.; Gadisseur, R.; Carlisi, A.; Monge, M.; Chapelle, J.-P.; Hubert, P.; Souberbielle, J.-C.; Delanaye, P. Measurement uncertainty of 25-OH vitamin D determination with different commercially available kits: Impact on the clinical cut offs. Osteoporos. Int. 2010, 21, 1047–1051. [Google Scholar] [CrossRef]
- College of American Pathologists. Measurement Uncertainty Guide ISO 15189 Accreditation Program. Available online: https://documents.cap.org/documents/cap15189-accreditation-program-measurement-uncertainty-guide.pdf (accessed on 25 November 2020).
- ISO-ISO/TS 20914:2019—Medical Laboratories—Practical Guidance for the Estimation of Measurement Uncertainty. Available online: https://www.iso.org/standard/69445.html (accessed on 21 January 2021).
- Durazo-Arvizu, R.; Ahmed, F.; Berry, J.; Cavalier, E.; Gunter, E.; Jones, G.; Jones, J.; Sempos, C.; Twomey, P.; Williams, E.; et al. Estimating uncertainty of target values for deqas serum materials. J. Steroid Biochem. Mol. Biol. 2019, 188, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Heijboer, A.C.; Blankenstein, M.A.; Kema, I.P.; Buijs, M.M. Accuracy of 6 routine 25-hydroxyvitamin D assays: Influence of vitamin D binding protein concentration. Clin. Chem. 2012, 58, 543–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depreter, B.; Heijboer, A.C.; Langlois, M.R. Accuracy of three automated 25-hydroxyvitamin D assays in hemodialysis patients. Clin. Chim. Acta 2013, 415, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Moreau, E.; Bächer, S.; Mery, S.; Le Goff, C.; Piga, N.; Vogeser, M.; Hausmann, M.; Cavalier, E. Performance characteristics of the VIDAS® 25-OH Vitamin D Total assay-comparison with four immunoassays and two liquid chromatography-tandem mass spectrometry methods in a multicentric study. Clin. Chem. Lab. Med. 2016, 54, 45–53. [Google Scholar] [CrossRef] [PubMed]



| Dataset | Subgroups | n Subject | n Results | Sample/ Subject | Replicates * | Mean Value, ng/mL (95% CI) | CVA (%) | CVI (%) | CVG (%) | n Results Eliminated % |
|---|---|---|---|---|---|---|---|---|---|---|
| Original data | 90 | 1696 | 9.52 | 1.96 | 19.0 (18.6–19.4) | 1.7 (1.6–1.8) | 17.8 | 1 subject for Vitamin D consumption from Padua, Italy | ||
| All results from 10 collections | All data | 46 | 813 | 8.93 | 1.96 | 22.5 (21.9–23.0) | 1.5 (1.4–1.6) | 6.3 (5.9–6.8) | 875 (51.8%) | |
| Males | 14 | 242 | 8.71 | 1.97 | 20.5 (19.7–20.8) | 6.2 (5.4–7.2) | 18.6 (14.1–32.2) | |||
| Females | 32 | 571 | 9.03 | 1.95 | 23.4 (22.7–24.2) | 6.4 (5.8–7.0) | 36.2 (28.6–49.2) | |||
| First set of 5 collections (1st–5th) | All data | 79 | 744 | 4.75 | 1.97 | 18.1 (17.5–18.7) | 1.9 (1.8–2.1) | 6.6 (6.1–7.2) | 114 (13.3%) | |
| Males | 33 | 309 | 4.70 | 1.99 | 15.6 (15.0–16.3) | 5.8 (5.1–6.7) | 37.1 (28.9–49.5) | |||
| Females | 46 | 435 | 4.78 | 1.96 | 19.9 (19.0–20.8) | 7.1 (6.5–8.0) | 51.1 (41.3–66.3) | |||
| Second set of 5 collections (6th–10th) | All data | 81 | 729 | 4.53 | 1.97 | 21.5 (20.9–22.1) | 1.5 (1.4–1.7) | 6.2 (5.7–6.7) | 109 (13.0%) | |
| Males | 33 | 289 | 4.39 | 1.99 | 19.4 (18.8–20.0) | 6.7 (5.9–7.8) | 25.0 (20.1–35.1) | |||
| Females | 48 | 440 | 4.63 | 1.96 | 22.8 (22.0–23.7) | 5.8 (5.2–6.4) | 39.2 (33.4–52.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalier, E.; Fraser, C.G.; Bhattoa, H.P.; Heijboer, A.C.; Makris, K.; Ulmer, C.Z.; Vesper, H.W.; Vasikaran, S.; Lukas, P.; Delanaye, P.; et al. Analytical Performance Specifications for 25-Hydroxyvitamin D Examinations. Nutrients 2021, 13, 431. https://doi.org/10.3390/nu13020431
Cavalier E, Fraser CG, Bhattoa HP, Heijboer AC, Makris K, Ulmer CZ, Vesper HW, Vasikaran S, Lukas P, Delanaye P, et al. Analytical Performance Specifications for 25-Hydroxyvitamin D Examinations. Nutrients. 2021; 13(2):431. https://doi.org/10.3390/nu13020431
Chicago/Turabian StyleCavalier, Etienne, Callum G. Fraser, Harjit P. Bhattoa, Annemieke C. Heijboer, Konstantinos Makris, Candice Z. Ulmer, Hubert W. Vesper, Samuel Vasikaran, Pierre Lukas, Pierre Delanaye, and et al. 2021. "Analytical Performance Specifications for 25-Hydroxyvitamin D Examinations" Nutrients 13, no. 2: 431. https://doi.org/10.3390/nu13020431
APA StyleCavalier, E., Fraser, C. G., Bhattoa, H. P., Heijboer, A. C., Makris, K., Ulmer, C. Z., Vesper, H. W., Vasikaran, S., Lukas, P., Delanaye, P., Carobene, A., & on behalf of the IFCC-IOF Committee for Bone Metabolism. (2021). Analytical Performance Specifications for 25-Hydroxyvitamin D Examinations. Nutrients, 13(2), 431. https://doi.org/10.3390/nu13020431