Genetic Variation in the Bitter Receptors Responsible for Epicatechin Detection Are Associated with BMI in an Elderly Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Data Collection
2.2. Blood Samples and BMI
2.3. Genotyping
2.4. Epicatechin Intake
2.5. Statistics
3. Results
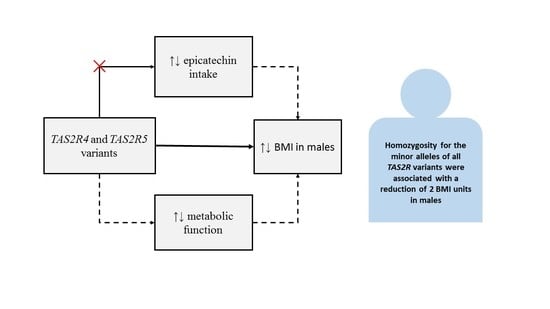
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grzesik, M.; Naparlo, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Ohyama, K.; Furuta, C.; Nogusa, Y.; Nomura, K.; Miwa, T.; Suzuki, K. Catechin-Rich Grape Seed Extract Supplementation Attenuates Diet-Induced Obesity in C57BL/6J Mice. Ann. Nutr. Metab. 2011, 58, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Phung, O.J.; Baker, W.L.; Matthews, L.J.; Lanosa, M.; Thorne, A.; Coleman, C.I. Effect of green tea catechins with or without caffeine on anthropometric measures: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2009, 91, 73–81. [Google Scholar] [CrossRef] [Green Version]
- De Los Santos, S.; Reyes-Castro, L.A.; Coral-Vazquez, R.M.; Mendez, J.P.; Leal-Garcia, M.; Zambrano, E.; Canto, P. (-)-Epicatechin reduces adiposity in male offspring of obese rats. J. Dev. Orig. Health Dis. 2020, 11, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A.; O’Donnell, R.; Shurpin, M.; Kordunova, D. Epicatechin, procyanidins, cocoa, and appetite: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Salmeán, G.; Ortiz-Vilchis, P.; Vacaseydel, C.M.; Rubio-Gayosso, I.; Meaney, E.; Villarreal, F.; Ramírez-Sánchez, I.; Ceballos, G. Acute effects of an oral supplement of (-)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects. Food Funct. 2014, 5, 521–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Prevalence of overweight among adults, BMI ≥ 25 (crude estimate) (%). Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-overweight-among-adults-bmi-greaterequal-25-(crude-estimate)-(-) (accessed on 12 November 2020).
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Osher, E.; Stern, N. Obesity in Elderly Subjects: In Sheep’s Clothing Perhaps, but still a Wolf! Diabetes Care 2009, 32, S398–S402. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.F.; Schatzkin, A.; Harris, T.B.; Kipnis, V.; Mouw, T.; Ballard-Barbash, R.; Hollenbeck, A.; Leitzmann, M.F. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N. Engl. J. Med. 2006, 355, 763–778. [Google Scholar] [CrossRef]
- Rillamas-Sun, E.; LaCroix, A.Z.; Waring, M.E.; Kroenke, C.H.; LaMonte, M.J.; Vitolins, M.Z.; Seguin, R.; Bell, C.L.; Gass, M.; Manini, T.M.; et al. Obesity and late-age survival without major disease or disability in older women. JAMA Intern. Med. 2014, 174, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, M.O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018, 6, 223–236. [Google Scholar] [CrossRef]
- Go, Y.; Satta, Y.; Takenaka, O.; Takahata, N. Lineage-specific loss of function of bitter taste receptor genes in humans and nonhuman primates. Genetics 2005, 170, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2Rbitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, J.; Yang, H.; Zhang, Y.-P. Adaptive Diversification of Bitter Taste Receptor Genes in Mammalian Evolution. Mol. Biol. Evol. 2003, 20, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Dotson, C.D.; Shaw, H.L.; Mitchell, B.D.; Munger, S.D.; Steinle, N.I. Variation in the gene TAS2R38 is associated with the eating behavior disinhibition in Old Order Amish women. Appetite 2010, 54, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Lee, J.; Yang, S.; Kim, J. Genetic variations in taste perception modify alcohol drinking behavior in Koreans. Appetite 2017, 113, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Diószegi, J.; Llanaj, E.; Ádány, R. Genetic Background of Taste Perception, Taste Preferences, and Its Nutritional Implications: A Systematic Review. Front. Genet. 2019, 10, 1272. [Google Scholar] [CrossRef] [Green Version]
- Perna, S.; Riva, A.; Nicosanti, G.; Carrai, M.; Barale, R.; Vigo, B.; Allegrini, P.; Rondanelli, M. Association of the bitter taste receptor gene TAS2R38 (polymorphism RS713598) with sensory responsiveness, food preferences, biochemical parameters and body-composition markers. A cross-sectional study in Italy. Int. J. Food Sci. Nutr. 2018, 69, 245–252. [Google Scholar] [CrossRef]
- Avau, B.; Rotondo, A.; Thijs, T.; Andrews, C.N.; Janssen, P.; Tack, J.; Depoortere, I. Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci. Rep. 2015, 5, 15985. [Google Scholar] [CrossRef]
- Janssen, S.; Laermans, J.; Verhulst, P.J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Wicks, D.; Wright, J.; Rayment, P.; Spiller, R. Impact of bitter taste on gastric motility. Eur. J. Gastroenterol. Hepatol. 2005, 17, 961–965. [Google Scholar] [CrossRef]
- Dotson, C.D.; Zhang, L.; Xu, H.; Shin, Y.K.; Vigues, S.; Ott, S.H.; Elson, A.E.; Choi, H.J.; Shaw, H.; Egan, J.M.; et al. Bitter taste receptors influence glucose homeostasis. PLoS ONE 2008, 3, e3974. [Google Scholar] [CrossRef] [Green Version]
- Cirera, S.; Clop, A.; Jacobsen, M.J.; Guerin, M.; Lesnik, P.; Jorgensen, C.B.; Fredholm, M.; Karlskov-Mortensen, P. A targeted genotyping approach enhances identification of variants in taste receptor and appetite/reward genes of potential functional importance for obesity-related porcine traits. Anim. Genet. 2018, 49, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, A.; Veysey, M.; Keely, S.; Scarlett, C.; Lucock, M.; Beckett, E.L. Interactions between Bitter Taste, Diet and Dysbiosis: Consequences for Appetite and Obesity. Nutrients 2018, 10, 1336. [Google Scholar] [CrossRef] [Green Version]
- Kim, U.K.; Jorgenson, E.; Coon, H.; Leppert, M.; Risch, N.; Drayna, D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science (N.Y.) 2003, 299, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and PROP Bitterness Depends on More Than the TAS2R38 Gene. Chem. Senses 2008, 33, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepper, B.J.; White, E.A.; Koelliker, Y.; Lanzara, C.; d’Adamo, P.; Gasparini, P. Genetic variation in taste sensitivity to 6-n-propylthiouracil and its relationship to taste perception and food selection. Ann. N Y Acad. Sci. 2009, 1170, 126–139. [Google Scholar] [CrossRef]
- Tepper, B.J.; Ullrich, N.V. Influence of genetic taste sensitivity to 6-n-propylthiouracil (PROP), dietary restraint and disinhibition on body mass index in middle-aged women. Physiol. Behav. 2002, 75, 305–312. [Google Scholar] [CrossRef]
- Choi, S.E.; Chan, J. Relationship of 6-n-propylthiouracil taste intensity and chili pepper use with body mass index, energy intake, and fat intake within an ethnically diverse population. J. Acad. Nutr. Dietetics 2015, 115, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.L.; Adise, S. Variation in the Ability to Taste Bitter Thiourea Compounds: Implications for Food Acceptance, Dietary Intake, and Obesity Risk in Children. Ann. Rev. Nutr. 2016, 36, 157–182. [Google Scholar] [CrossRef]
- Duffy, V.B. Associations between oral sensation, dietary behaviors and risk of cardiovascular disease (CVD). Appetite 2004, 43, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Aguera, Z.; Sabater, M.; Moreno-Navarrete, J.M.; Alonso-Ledesma, I.; Xifra, G.; Botas, P.; Delgado, E.; Jimenez-Murcia, S.; Fernandez-Garcia, J.C.; et al. Genetic variations of the bitter taste receptor TAS2R38 are associated with obesity and impact on single immune traits. Mol. Nutr. Food Res. 2016, 60, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H. Variation in the TAS2R38 Bitterness Receptor Gene Was Associated with Food Consumption and Obesity Risk in Koreans. Nutrients 2019, 11, 1973. [Google Scholar] [CrossRef] [Green Version]
- Tepper, B.J.; Koelliker, Y.; Zhao, L.; Ullrich, N.V.; Lanzara, C.; D’Adamo, P.; Ferrara, A.; Ulivi, S.; Esposito, L.; Gasparini, P. Variation in the Bitter-taste Receptor Gene TAS2R38, and Adiposity in a Genetically Isolated Population in Southern Italy. Obesity 2008, 16, 2289–2295. [Google Scholar] [CrossRef]
- Keller, K.L.; Reid, A.; MacDougall, M.C.; Cassano, H.; Song, J.L.; Deng, L.; Lanzano, P.; Chung, W.K.; Kissileff, H.R. Sex Differences in the Effects of Inherited Bitter Thiourea Sensitivity on Body Weight in 4–6-Year-Old Children. Obesity 2010, 18, 1194–1200. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, G.L.; Daun, H.; Tepper, B.J. Influence of PROP taster status and maternal variables on energy intake and body weight of pre-adolescents. Physiol. Behav. 2007, 90, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk-Stecyna, J.; Malinowska, A.M.; Chmurzynska, A. TAS2R38 and CA6 genetic polymorphisms, frequency of bitter food intake, and blood biomarkers among elderly woman. Appetite 2017, 116, 57–64. [Google Scholar] [CrossRef]
- Pawellek, I.; Grote, V.; Rzehak, P.; Xhonneux, A.; Verduci, E.; Stolarczyk, A.; Closa-Monasterolo, R.; Reischl, E.; Koletzko, B. Association of TAS2R38 variants with sweet food intake in children aged 1-6 years. Appetite 2016, 107, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, J.C.; Cardinal, T.M.; Sitto, J.R.; Kannan, S. Ability to taste 6-n-propylthiouracil and BMI in low-income preschool-aged children. Obesity 2008, 16, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Keller, K.L.; Tepper, B.J. Inherited Taste Sensitivity to 6-n-Propylthiouracil in Diet and Body Weight in Children. Obesity Res. 2004, 12, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Simchen, U.; Koebnick, C.; Hoyer, S.; Issanchou, S.; Zunft, H.J. Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. European J. Clin. Nutr. 2006, 60, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Roudnitzky, N.; Behrens, M.; Engel, A.; Kohl, S.; Thalmann, S.; Hübner, S.; Lossow, K.; Wooding, S.P.; Meyerhof, W. Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception. PLoS Genet. 2015, 11, e1005530. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.E.; Wallace, M.R.; Knopik, V.S.; Herbstman, D.M.; Bartoshuk, L.M.; Duffy, V.B. Allelic Variation in TAS2RBitter Receptor Genes Associates with Variation in Sensations from and Ingestive Behaviors toward Common Bitter Beverages in Adults. Chem. Senses 2010, 36, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.J.A.; Veysey, M.; Lucock, M.; Niblett, S.; King, K.; MacDonald-Wicks, L.; Garg, M.L. Association between omega-3 index and blood lipids in older Australians. J. Nutr. Biochem. 2016, 27, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Guilbert, C.; Keogh, J.; Syrette, J.; Lange, K.; Cox, D.N. Estimating food intakes in Australia: Validation of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) food frequency questionnaire against weighed dietary intakes. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2009, 22, 559–566. [Google Scholar] [CrossRef]
- Ward, S.J.; Coates, A.M.; Hill, A.M. Application of an Australian Dietary Guideline Index to Weighed Food Records. Nutrients 2019, 11, 1286. [Google Scholar] [CrossRef] [Green Version]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Beckett, E.L.; Martin, C.; Boyd, L.; Porter, T.; King, K.; Niblett, S.; Yates, Z.; Veysey, M.; Lucock, M. Reduced plasma homocysteine levels in elderly Australians following mandatory folic acid fortification – A comparison of two cross-sectional cohorts. J. Nutr. Intermed. Metab. 2017, 8, 14–20. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Australian Health Survey: Consumption of Food Groups from the Australian Dietary Guidelines. Available online: https://www.abs.gov.au/ausstats/[email protected]/Lookup/by%20Subject/4364.0.55.012~2011-12~Main%20Features~Key%20Findings~1 (accessed on 16 July 2020).
- Marfell-Jones, M.; Stewart, T.O.A.; Carter, L. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Potchefstroom, South Africa, 2006. [Google Scholar]
- Beckett, E.L.; Duesing, K.; Martin, C.; Jones, P.; Furst, J.; King, K.; Niblett, S.; Yates, Z.; Veysey, M.; Lucock, M. Relationship between methylation status of vitamin D-related genes, vitamin D levels, and methyl-donor biochemistry. J. Nutr. Intermed. Metab. 2016, 6, 8–15. [Google Scholar] [CrossRef] [Green Version]
- QIAGEN. QIAamp® DNA Mini and Blood Mini Handbook. Available online: https://www.qiagen.com/au/resources/resourcedetail?id=62a200d6-faf4-469b-b50f-2b59cf738962&lang=en (accessed on 23 April 2020).
- Thermo Fisher Scientific Inc. TaqMan® SNP Genotyping Assays USER GUIDE. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0009593_TaqManSNP_UG.pdf (accessed on 23 April 2020).
- Ferraris, C.; Turner, A.; Kaur, K.; Piper, J.; Veysey, M.; Lucock, M.; Beckett, E.L. Salt Taste Genotype, Dietary Habits and Biomarkers of Health: No Associations in an Elderly Cohort. Nutrients 2020, 12, 1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.C. Nutrition epidemiology of flavan-3-ols: The known unknowns. Mol. Asp. Med. 2018, 61, 2–11. [Google Scholar] [CrossRef]
- Kivimäki, M.; Kuosma, E.; Ferrie, J.E.; Luukkonen, R.; Nyberg, S.T.; Alfredsson, L.; Batty, G.D.; Brunner, E.J.; Fransson, E.; Goldberg, M.; et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017, 2, e277–e285. [Google Scholar] [CrossRef] [Green Version]
- Akil, L.; Ahmad, H.A. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. J. Health Care Poor Underserved 2011, 22, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Tirosh, A.; Shai, I.; Afek, A.; Dubnov-Raz, G.; Ayalon, N.; Gordon, B.; Derazne, E.; Tzur, D.; Shamis, A.; Vinker, S.; et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N. Engl. J. Med. 2011, 364, 1315–1325. [Google Scholar] [CrossRef] [Green Version]
- Jeon, T.-I.; Zhu, B.; Larson, J.L.; Osborne, T.F. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J. Clin. Investig. 2008, 118, 3693–3700. [Google Scholar] [CrossRef] [Green Version]
- Monica, C.; Chen, S.; Wu, V.; Joseph, R.; Reeve, J.; Rozengurt, E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: Role of L-type voltage-sensitive Ca2+ channels. Am. J. Physiol. Cell Physiol. 2006, 291, C726–C739. [Google Scholar] [CrossRef] [Green Version]
- Depoortere, I. Taste receptors of the gut: Emerging roles in health and disease. Gut 2014, 63, 179–190. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirch, N.; Berk, L.; Liegl, Y.; Adelsbach, M.; Zimmermann, B.F.; Stehle, P.; Stoffel-Wagner, B.; Ludwig, N.; Schieber, A.; Helfrich, H.-P.; et al. A nutritive dose of pure (–)-epicatechin does not beneficially affect increased cardiometabolic risk factors in overweight-to-obese adults—a randomized, placebo-controlled, double-blind crossover study. Am. J. Clin. Nutr. 2018, 107, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Beckett, E.L.; Duesing, K.; Boyd, L.; Yates, Z.; Veysey, M.; Lucock, M. A potential sex dimorphism in the relationship between bitter taste and alcohol consumption. Food Funct. 2017, 8, 1116–1123. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, J.; Yang, S.; Lee, E.K.; Hwangbo, Y.; Kim, J. Genetic variations in TAS2R3 and TAS2R4 bitterness receptors modify papillary carcinoma risk and thyroid function in Korean females. Sci. Rep. 2018, 8, 15004. [Google Scholar] [CrossRef]
- Boyce, J.M.; Shone, G.R. Effects of ageing on smell and taste. Postgrad. Med. J. 2006, 82, 239–241. [Google Scholar] [CrossRef] [Green Version]
- Sergi, G.; Bano, G.; Pizzato, S.; Veronese, N.; Manzato, E. Taste loss in the elderly: Possible implications for dietary habits. Crit. Rev. Food Sci. Nutr. 2017, 57, 3684–3689. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]



| Groups | High-Epicatechin Foods | Average mg/100 g [58] | SD | Standard Serving Size [52] | Average mg/Serve |
|---|---|---|---|---|---|
| Tea | Tea [Green], infusion | 7.9 | 13.7 | 200 mL | 15.9 |
| Tea [Black], infusion | 3.9 | 4.3 | 200 mL | 7.9 | |
| Chocolate | Chocolate, dark | 70.3 | 29.5 | 25 g | 17.7 |
| Chocolate, milk | 14.6 | 4.8 | 25 g | 3.7 | |
| Wine | Wine [Red] | 3.8 | 3.2 | 100 mL | 3.8 |
| Wine [White] | 1.0 | 1.4 | 100 mL | 1.0 | |
| Fruits | Apple [Dessert], raw | 8.4 | 3.7 | 150 g | 12.6 |
| Peach, peeled | 8.0 | 4.2 | 150 g | 12.0 | |
| Apple [Dessert], pure juice | 7.8 | 7.7 | 150 g | 11.6 | |
| Grape [Black] | 5.2 | 5.6 | 150 g | 7.9 | |
| Red raspberry, raw | 5.1 | 3.7 | 150 g | 7.6 | |
| Apricot, raw | 3.5 | 4.3 | 150 g | 5.2 | |
| Nectarine, peeled | 3.0 | 1.1 | 150 g | 4.5 | |
| Plum, fresh | 2.2 | 2.2 | 150 g | 3.3 | |
| Blueberry, raw | 1.1 | 0 | 150 g | 1.7 | |
| Grape [Green] | 0.5 | 0.5 | 150 g | 0.7 | |
| Avocado, raw | 0.4 | 0.2 | 150 g | 0.6 | |
| Kiwi | 0.3 | 0.2 | 150 g | 0.4 | |
| Banana, raw | 0.1 | 0.1 | 150 g | 0.2 | |
| Vegetables | Broad bean seed, raw | 22.5 | 0 | 75 g | 16.9 |
| Green bean, raw | 0.7 | 2.7 | 75 g | 0.5 | |
| Nuts | Lentils, whole, raw | 0.1 | 0.3 | 75 g | 0.1 |
| Cashew nut, raw | 0.9 | 0 | 30 g | 0.3 | |
| Pecan nut | 0.8 | 0 | 30 g | 0.2 | |
| Almond | 0.6 | 0.4 | 30 g | 0.2 | |
| Hazelnut, raw | 0.2 | 0 | 30 g | 0.1 |
| Characteristic | Male (n = 254) | Female (n = 309) | Total (n = 563) |
|---|---|---|---|
| Age | 77.4 (76.6–78.3) | 77.3 (76.6–78.2) | 77.4 (76.8–78.0) |
| BMI | 28.5 (27.9–29.1) | 28.6 (28.0–29.2) | 28.5 (28.1–29.0) |
| Daily energy intake (kJ) | * 8656.2 (8311.3–9001.1) | * 7866.7 (7563.1–8170.3) | 8223.5 (8453.1–7993.9) |
| Serves of high-epicatechin foods per day ** | 5.2 (4.9–5.6) | 5.2 (4.9–5.5) | 5.2 (5.0–5.4) |
| SNP | Genotype | n | % | MAF | HWE χ2 | HWE p |
|---|---|---|---|---|---|---|
| TAS2R4 (rs2233998) | CC | 112 | 21% | 0.42 | 3.8 | 0.05 |
| CT | 287 | 54% | - | |||
| TT | 131 | 25% | - | |||
| TAS2R4 (rs2234001) | CC | 108 | 20% | 0.48 | 121.8 | <0.0001 |
| CG | 302 | 55% | - | |||
| GG | 135 | 25% | - | |||
| TAS2R5 (rs2227264) | TT | 120 | 21% | 0.44 | 0.8 | 0.4 |
| TG | 291 | 62% | - | |||
| GG | 151 | 27% | - |
| SNP | TAS2R4 (rs2233998) | TAS2R4 (rs2234001) | TAS2R5 (rs2227264) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CC | CT/TT | p | CC | CG/GG | p | TT | TG/GG | p | |
| Mean kJ/day (95% CI) | 7934.5 (7428.0–8441.0) | 8278.6 (8013.4–8543.7) | 0.2 | 7905.2 (7386.8–8423.7) | 8272.3 (8011.8–8532.8) | 0.2 | 8056.8 (7564.0–8549.6) | 8304.7 (8045.3–8564.2) | 0.4 |
| Male mean kJ/day(95% CI) | 8530.8 (7777.1–9284.6) | 8644.6 (8236.8–9052.4) | 0.8 | 8552.8 (7794.8–9310.9) | 8585.0 (8189.5–8980.6) | 0.9 | 8574.6 (7834.9–9314.3) | 8686.0 (8288.6–9083.4) | 0.8 |
| Female mean kJ/day (95% CI) | 7350.2 (6660.4–8039.9) | 7902 (7554.9–8249.3) | 0.1 | 7282.3 (6565.9–7998.7) | 7943.7 (7598.9–8288.4) | 0.1 | 7561.5 (6896.1–8226.9) | 7914.7 (7573.4–8256.0) | 0.4 |
| SNP | TAS2R4 (rs2233998) | p | TAS2R4 (rs2234001) | p | TAS2R5 (rs2227264) | p | |||
|---|---|---|---|---|---|---|---|---|---|
| CC | CT/TT | CC | CG/GG | TT | TG/GG | ||||
| Mean (95% CI) | 5.3 (4.8–5.7) | 5.2 (4.9–5.4) | 0.7 | 5.3 (4.8–5.8) | 5.2 4.9–5.4) | 0.8 | 5.4 (4.9–5.9) | 5.2 (4.9–5.5) | 0.5 |
| Male mean (95% CI) | 5.5 (4.8–6.3) | 5.1 (4.7–5.5) | 0.3 | 5.4 (4.7–6.1) | 5.1 (4.7–5.5) | 0.5 | 5.6 (4.8–6.3) | 5.2 (4.8–5.6) | 0.4 |
| Female mean (95% CI) | 5.0 (4.2–5.7) | 5.2 (4.8–5.6) | 0.6 | 5.1 (4.4–5.8) | 5.2 (4.9–5.6) | 0.7 | 5.2 (4.5–5.9) | 5.2 (4.9–5.6) | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, A.; Veysey, M.; Keely, S.; Scarlett, C.J.; Lucock, M.; Beckett, E.L. Genetic Variation in the Bitter Receptors Responsible for Epicatechin Detection Are Associated with BMI in an Elderly Cohort. Nutrients 2021, 13, 571. https://doi.org/10.3390/nu13020571
Turner A, Veysey M, Keely S, Scarlett CJ, Lucock M, Beckett EL. Genetic Variation in the Bitter Receptors Responsible for Epicatechin Detection Are Associated with BMI in an Elderly Cohort. Nutrients. 2021; 13(2):571. https://doi.org/10.3390/nu13020571
Chicago/Turabian StyleTurner, Alexandria, Martin Veysey, Simon Keely, Christopher J. Scarlett, Mark Lucock, and Emma L. Beckett. 2021. "Genetic Variation in the Bitter Receptors Responsible for Epicatechin Detection Are Associated with BMI in an Elderly Cohort" Nutrients 13, no. 2: 571. https://doi.org/10.3390/nu13020571
APA StyleTurner, A., Veysey, M., Keely, S., Scarlett, C. J., Lucock, M., & Beckett, E. L. (2021). Genetic Variation in the Bitter Receptors Responsible for Epicatechin Detection Are Associated with BMI in an Elderly Cohort. Nutrients, 13(2), 571. https://doi.org/10.3390/nu13020571