Assessment of Health Claims Related to Folic Acid in Food Supplements for Pregnant Women According to the European Regulation
Abstract
:1. Introduction
1.1. Food Supplements’ Safety: An Overview of the State of the Art
1.2. Health-Related Claims of Food Supplements: Regulatory Framework in Europe and Its Implications for Consumers Health
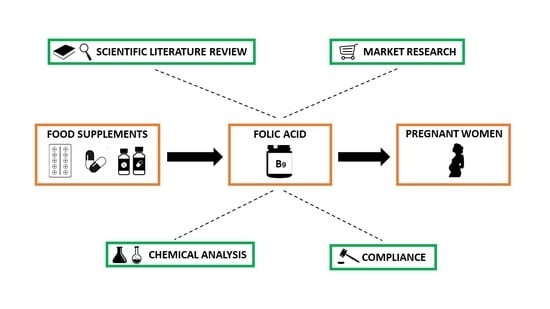
2. Materials and Methods
2.1. Scientific Literature Review
2.2. Food Supplements Market Research
2.3. Analytical Determination of Folic Acid
Statistical Analysis
3. Results and Discussion
3.1. Scientific Literature Review of Health-Related Claims Approved for Folic Acid in the European Regulation
3.2. Food Supplements Market Research Results
3.3. Sample Selection for Chemical Analysis
3.4. Analytical Determination of Folic Acid and Assessment of the Level of Compliance of Its Health-Related Claims
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- European Parliament and Council of the European Union. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off. J. Eur. Union 2002, L183, 51. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32002L0046 (accessed on 21 December 2020).
- European Food Safety Authority (EFSA). Food Supplements—Introduction. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 20 December 2020).
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. The frontier between nutrition and pharma: The international regulatory framework of functional foods, food supplements and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2019, 60, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Bureau Européen des Unions de Consommateurs (BEUC). Food Supplements. Challenges & Risks for Consumers; BEUC-X-2016-092; BEUC Publications: Brussels, Belgium, 2016. [Google Scholar]
- Funnell, G.; Naicker, K.; Chang, J.; Hill, N.; Kayyali, R. A cross-sectional survey investigating women’ information sources, behaviour, expectations, knowledge and level of satisfaction on advice received about diet and supplements before and during pregnancy. BMC Pregnancy Childbirth 2018, 18, 182. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Gahche, J.J.; Potischman, N.; Dwyer, J.T.; Guenther, P.M.; Suader, K.A.; Bailey, R.L. Dietary Supplement Use and Its Micronutrient Contribution During Pregnancy and Lactation in the United States. Obstet. Gynecol. 2020, 135, 623–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Good Maternal Nutrition. The Best Start in Life; WHO Regional Office for Europe: Copenhagen, Denmark, 2016; ISBN 978 92 890 5154 5. [Google Scholar]
- Koletzko, B.; Cremer, M.; Flothkötter, M.; Graf, C.; Hauner, H.; Hellmers, C.; Kersting, M.; Krawinkel, M.; Przyrembel, H.; Röbl-Mathieu, M.; et al. Diet and Lifestyle Before and During Pregnancy—Practical Recommendations of the Germany-wide Healthy Start—Young Family Network. Geburtsh Frauenheilk 2018, 78, 1262–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meija, L.; Rezeberga, D.; Proper Maternal Nutrition during Pregnancy Planning and Pregnancy: A Healthy Start in Life Recommendations for Health Care Professionals—The Experience from Latvia. Recommendations for Health Care Specialists; 2017. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/publications/2017/proper-maternal-nutrition-during-pregnancy-planning-and-pregnancy-a-healthy-start-in-life-2017 (accessed on 17 December 2020).
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, E.M.; Grimshaw, K.E.C.; Schoemaker, A.A.; Keil, T.; McBride, D.; Sprikkelman, A.B.; Ragnarsdottir, H.S.; Trendelenburg, V.; Emmanouil, E.; Reche, M.; et al. Dietary Habits and Supplement Use in Relation to National Pregnancy Recommendations: Data from the EuroPrevall Birth Cohort. Matern. Child Health J. 2014, 18, 2408–2425. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Food Supplements. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/supplements_en (accessed on 18 December 2020).
- Brown, B.; Wright, C. Safety and efficacy of supplements in pregnancy. Nutr. Rev. 2020, 78(10), 813–826. [Google Scholar] [CrossRef] [PubMed]
- European Commission. RASFF—Food and Feed Safety Alerts. Available online: https://ec.europa.eu/food/safety/rasff_en (accessed on 12 December 2020).
- European Commission. RASFF Portal. Available online: https://webgate.ec.europa.eu/rasff-window/portal/?event=searchResultList&StartRow=1 (accessed on 18 December 2020).
- National Food Institute—Technical University of Denmark (DTU). Many Danes Take Dietary Supplements although Few Need Them. Available online: https://www.food.dtu.dk/english/news/2016/03/many-danes-take-dietary-supplements-although-few-need-them?id=f9017469-c293-4f28-bd71-163a491a4ae8 (accessed on 15 December 2020).
- German Federal Institute for Risk Assessment (BfR). German Test Suggests “Many” Vitamin Supplements Exceed Safe Dose Limits. Available online: https://www.nutraingredients.com/Article/2017/09/07/German-test-suggests-many-vitamin-supplements-exceed-safe-dose-limits?utm_source=copyright&utm_medium=OnSite&utm_campaign=copyright (accessed on 16 December 2020).
- European Parliament and Council of the European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on food. Off. J. Eur. Union 2006, L404, 9. Available online: http://eurlex. europa.eu/legal-content/EN/ALL/?uri=CELEX%3A02006R1924-20121129 (accessed on 22 December 2020).
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Off. J. Eur. Union 2011, L304, 18. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32011R1169 (accessed on 22 December 2020).
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Dorta, E.; Maher, S.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Sánchez-Mata, M.C. Potential Nutrition and Health Claims in Deastringed Persimmon Fruits (Diospyros kaki L.), Variety ‘Rojo Brillante’, PDO ‘Ribera del Xúquer’. Nutrients 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Getov, I.N.; Lebanova, H.; Grigorov, E. Food supplements—marketing and health claims analysis. Arch. Balk. Med Union 2011, 46, 26–29. [Google Scholar] [CrossRef]
- U.S. Pharmacopeia (USP). Ensuring the Quality of Dietary Supplements. OTH430F_2016-02. 2016. Available online: https://www.usp.org/sites/default/files/usp/document/about/public-policy/public-policy-dietary-supplements.pdf (accessed on 18 December 2020).
- European Commission. EU Register of Nutrition and Health Claims Made on Food. Available online: http://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=register.home (accessed on 18 December 2020).
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Informes del Sistema Coordinado de Intercambio de Información (SCIRI). Available online: http://www.aecosan.msssi.gob.es/AECOSAN/web/seguridad_alimentaria/subseccion/SCIRI.htm (accessed on 15 December 2020).
- Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Tardío, J. Optimization and Application of FL-HPLC for Folates Analysis in 20 Species of Mediterranean Wild Vegetables. Food Anal. Methods 2015, 8, 302–311. [Google Scholar] [CrossRef]
- Matias, R.; Ribeiro, P.R.S.; Sarraguça, M.C.; Lopes, J.A. A UV spectrophotometric method for the determination of folic acid in pharmaceutical tablets and dissolution tests. Anal. Methods 2014, 6, 3065. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the substantiation of health claims related to folate and blood formation, homocysteine metabolism, energy-yielding metabolism, function of the immune system, function of blood vessels, cell division, and maternal tissue growth during pregnancy pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1213. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2009.1213 (accessed on 22 December 2020).
- European Food Safety Authority (EFSA). Scientific Opinion on the substantiation of health claims related to folate and contribution to normal psychological functions, maintenance of normal vision, reduction of tiredness and fatigue, cell division and contribution to normal amino acid synthesis pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1760. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2010.1760 (accessed on 21 December 2020).
- European Food Safety Authority (EFSA). Scientific Opinion on the substantiation of a health claim related to increasing maternal folate status by supplemental folate intake and reduced risk of neural tube defects pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2013, 11, 3328. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2013.3328 (accessed on 21 December 2020).
- European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on food, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L136, 1. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32012R0432 (accessed on 22 December 2020).
- European Commission. Guidance Document for Competent Authorities for the Control of Compliance with EU Legislation on Regulation (EU) No 1169/2011, Council Directive 90/496/EEC and Directive 2002/46/EC with Regard to the Setting of Tolerances for Nutrient Values Declared on a Label. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/labelling_legislation/nutrition-labelling_en (accessed on 13 December 2020).
| Model Claim | European Food Safety Authority (EFSA) Opinion Reference | |
|---|---|---|
| Health claims | ||
| “Folate contributes to maternal tissue growth during pregnancy” | [28] | |
| “Folate contributes to normal amino acid synthesis” | [29] | |
| “Folate contributes to normal blood formation” | [28] | |
| “Folate contributes to normal homocysteine metabolism” | [28] | |
| “Folate contributes to normal psychological function” | [29] | |
| “Folate contributes to the normal function of the immune system” | [28] | |
| “Folate contributes to the reduction of tiredness and fatigue” | [29] | |
| “Folate has a role in the process of cell division” | [28,29] | |
| Use conditions: Food and food supplements which are at least a “Source of folate” according to the requirements established in the Annex to Regulation (EC) Nº 1924/2006 | ||
| Reduction of disease risk claim | ||
| “Supplemental folic acid intake increases maternal folate status. Low maternal folate status is a risk factor in the development of neural tube defects in the developing fetus” | [30] | |
| Use conditions: Food supplements that provide at least 400 µg of folic acid/day. The target population is women of child-bearing age, and the beneficial effect is obtained with a supplemental folic acid daily intake of 400 µg for at least one month before and up to three months after conception | ||
| Source | Total Number | Food Supplements with Approved Health Claims Related to Folic Acid | Food Supplements with Unapproved Health Claims Related to Folic Acid |
|---|---|---|---|
| In situ purchase | 11 | 11 | 0 |
| Online purchase | 70 | 60 | 10 |
| Inclusion Criterion 1 Target Population: Pregnant Women | Inclusion Criterion 2 “Reduction of Disease Risk” Claim Referred to Folic Acid | Inclusion Criterion 3 Health Claims Related to Vitamins and Minerals | |
|---|---|---|---|
| Total | 71 | 12 | 4 |
| FS1 | ✓ | ✓ | 23 health claims Vitamins: B6, B9, B12, C, D Minerals: Cu, Fe, Zn, Ca, Mg, Se, I |
| FS2 | ✓ | ✓ | 4 health claims Vitamins: B9, D |
| FS3 | ✓ | ✓ | 2 health claims Minerals: Mn, Zn |
| FS4 | ✓ | ✓ | 5 health claims Vitamins: D Minerals: Fe, Ca, I |
| Food Supplement Sample | Value Declared in the Labeling (µg/sachet) | Range of Tolerance (RT) (µg/sachet) | Analytical Value (µg/sachet) |
|---|---|---|---|
| FS1 | 500 | 399.60–750.60 | 509.55 ± 9.92 |
| FS2 | 400 | 390.03–600.60 | 408.59 ± 9.47 |
| FS3 | 400 | 396.25–600.60 | 429.52 ± 3.25 |
| FS4 | 200 | 159.60–300.60 | 220.69 ± 9.09 |
| Health Claims | Reduction of Disease Risk Claim | |||
|---|---|---|---|---|
| Sample | Declared Value (labeling) | Analytical Value (µg/sachet) | Legal Requirement to Use the Claims | Legal Requirement to Use the Claim |
| FS1 | 500 µg/sachet 1 sachet = 14 g | 509.55 ± 9.92 | Food supplements must be a “Source of folate”, that is, 15% of the Nutrient Reference Value (NRV) of folic acid is supplied by 100 g NRV of folic acid = 200 µg/100 g 15% NRV = 30 µg/100 g | Food supplements must provide at least 400 µg of folic acid/day. |
| FS2 | 400 µg/sachet 1 sachet = 4.02 g | 408.59 ± 9.47 | ||
| FS3 | 400 µg/sachet 1 sachet = 2.3 g | 429.52 ± 3.25 | ||
| FS4 | 200 µg/sachet 1 sachet = 6 g | 220.69 ± 9.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez, L.; Fernández-Ruiz, V.; Morales, P.; Sánchez-Mata, M.-C.; Cámara, M. Assessment of Health Claims Related to Folic Acid in Food Supplements for Pregnant Women According to the European Regulation. Nutrients 2021, 13, 937. https://doi.org/10.3390/nu13030937
Domínguez L, Fernández-Ruiz V, Morales P, Sánchez-Mata M-C, Cámara M. Assessment of Health Claims Related to Folic Acid in Food Supplements for Pregnant Women According to the European Regulation. Nutrients. 2021; 13(3):937. https://doi.org/10.3390/nu13030937
Chicago/Turabian StyleDomínguez, Laura, Virginia Fernández-Ruiz, Patricia Morales, María-Cortes Sánchez-Mata, and Montaña Cámara. 2021. "Assessment of Health Claims Related to Folic Acid in Food Supplements for Pregnant Women According to the European Regulation" Nutrients 13, no. 3: 937. https://doi.org/10.3390/nu13030937
APA StyleDomínguez, L., Fernández-Ruiz, V., Morales, P., Sánchez-Mata, M. -C., & Cámara, M. (2021). Assessment of Health Claims Related to Folic Acid in Food Supplements for Pregnant Women According to the European Regulation. Nutrients, 13(3), 937. https://doi.org/10.3390/nu13030937