Fasting: How to Guide
Abstract
:1. Introduction
1.1. Historical Background
1.2. Fasting as a Choice for Longevity and Health Benefits
2. Fasting Response in Different Organisms
Metabolic and Biochemical Effects of Starvation in Humans
3. Fasting Physiology and Models
3.1. Physiology of Fasting
3.2. Types of Fasting
3.3. Metabolic and Physiological Responses in Animal and Human Models
3.3.1. Effects of TRF
3.3.2. IF: Effects of ADF and MADF
3.3.3. Effects of PF
4. Is Fasting Safe? Body Composition and Nutritional Status Emendation
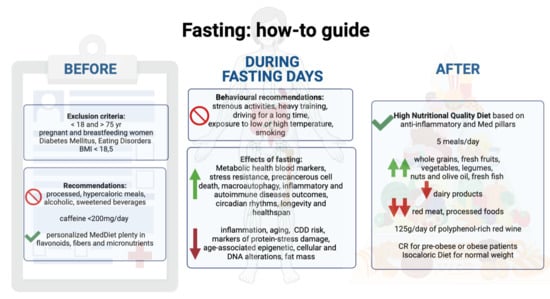
5. Guidelines
5.1. Inclusion and Exclusion Criteria
5.2. General Recommendations
5.3. Behavioral Recommendations during Fasting Days
5.4. Eating Recommendation before and after Fasting Days
6. Recommendation in Patients with Diabetes Mellitus
7. Recommendation in Patients with Cardiovascular Diseases
8. Recommendation for Patients with Cancer
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CR | Caloric Restriction |
| CDD | Chronic Degenerative Diseases |
| ADF | Alternate Day Fasting |
| LDL-Cholesterol | Low Density Lipoprotein Cholesterol |
| HDL | High Density Lipoprotein |
| TOR-S6k | Target of Rapamycin-S6 Kinase |
| PKA | Protein Kinase A |
| Ras2 | Rat Sarcoma 2 |
| TOR1 | Target of Rapamicin 1 |
| Sch9 | Serin/Threonin-Protein kinase |
| Msn/4 | heterodimeric zinc-finger transcription factor |
| Gis1 | Transcriptional activator/repressor GIS1 |
| GTPase RHEB-1 | Regulatory Protein G of mTOR |
| mTORC1 | Mechanistic Target of Rapamicyn Complex 1 |
| IIS | insulin/IGF (insulin-like growth factor)-like signalling |
| IGF1 | Insulin Growth Factor 1 |
| AD | Alzheimer Disease |
| DNA | Deoxy Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| CAT | Catalase |
| SOD | Superoxide Dismutase |
| GPx | Glutathione Peroxidase |
| Aβ | Amiloide Beta |
| mTor | Mechanistic Target of Rapamicyn |
| mTORC2 | Mechanistic Target of Rapamicyn Complex 2 |
| TRF | Time Restricted Feeding |
| IF | Intermittent Fasting |
| MADF | Modified Alternate Day Fasting |
| HF diet | High Fat diet |
| TG | Triglycerides |
| PF | Prolonged Fasting |
| BDNF | Brain-Derived Neurotrophic Factor |
| NPC | Neural Precursor Cells |
| BMI | Body Mass Index |
| FFM | Free Fat Mass |
| RT | Resistance Training |
| ASMMI | Appendicular Skeletal Muscle Mass Index |
| BCMI | Body Cellular Mass Index |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| CO | Carbon Monoxide |
| COPD | Chronic Obstructive Pulmonary Disease |
| HNQD | High Nutritional Quality Diet |
| MUFA | Mono-Unsaturated Fatty Acids |
| PUFA | Poly Unsaturated Fatty Acids |
| LM | Lean Mass |
| T2D | Type 2 Diabetes |
| HbA1c | Glycated Haemoglobin |
| T1D | Type 1 Diabetes |
| Sox2 | SRY (sex determining region Y)-box 2 |
| Ngn3 | Neurogenin3 |
| MI | Myocardial Infarction |
References
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.C. Fasting as a Therapy in Neurological Disease. Nutrients 2019, 11, 2501. [Google Scholar] [CrossRef] [Green Version]
- Galassi, F.M.; Bender, N.; Habicht, M.E.; Armocida, E.; Toscano, F.; Menassa, D.A.; Cerri, M. St. Catherine of Siena (1347–1380 AD): One of the earliest historic cases of altered gustatory perception in anorexia mirabilis. Neurol. Sci. 2018, 39, 939–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, J. Sir William Withey Gull (1816–1890). Eur. Neurol. 2006, 55, 53–56. [Google Scholar] [CrossRef]
- Harris, J.C. Anorexia Nervosa and Anorexia Mirabilis. JAMA Psychiatry 2014, 71, 1212–1213. [Google Scholar] [CrossRef] [PubMed]
- Persynaki, A.; Karras, S.; Pichard, C. Unraveling the metabolic health benefits of fasting related to religious beliefs: A narrative review. Nutrition 2017, 35, 14–20. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Bloomer, R.J. The impact of religious fasting on human health. Nutr. J. 2010, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, W.H.; Jarrar, A.H.; Al Baz, S.A.; Habib, H.M. Effect of Ramadan Fasting on Markers of Oxidative Stress and Serum Biochemical Markers of Cellular Damage in Healthy Subjects. Ann. Nutr. Metab. 2008, 53, 175–181. [Google Scholar] [CrossRef]
- Lessan, N.; Ali, T. Energy Metabolism and Intermittent Fasting: The Ramadan Perspective. Nutrients 2019, 11, 1192. [Google Scholar] [CrossRef] [Green Version]
- Sarri, K.; E Tzanakis, N.; Linardakis, M.K.; Mamalakis, G.D.; Kafatos, A.G. Effects of Greek orthodox christian church fasting on serum lipids and obesity. BMC Public Health 2003, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Papadaki, A.; Vardavas, C.; Hatzis, C.; Kafatos, A. Calcium, nutrient and food intake of Greek Orthodox Christian monks during a fasting and non-fasting week. Public Health Nutr. 2008, 11, 1022–1029. [Google Scholar] [CrossRef] [Green Version]
- Bloomer, R.J.; Kabir, M.M.; E Canale, R.; Trepanowski, J.F.; E Marshall, K.; Farney, T.M.; Hammond, K.G. Effect of a 21 day Daniel Fast on metabolic and cardiovascular disease risk factors in men and women. Lipids Health Dis. 2010, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creamer, D. Malnutrition and skin disease in Far East prisoners-of-war in World War II. Clin. Exp. Dermatol. 2018, 43, 766–769. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, F.A.; B., M.; Keys, A.; Brozek, J.; Henschel, A.; Mickelsen, O.; Taylor, H.L. The Biology of Human Starvation. Am. J. Psychol. 1951, 64, 292. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Obert, J.; Pearlman, M.; Obert, L.; Chapin, S. Popular Weight Loss Strategies: A Review of Four Weight Loss Techniques. Curr. Gastroenterol. Rep. 2017, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [Green Version]
- Longo, V.D.; Antebi, A.; Bartke, A.; Barzilai, N.; Brown-Borg, H.M.; Caruso, C.; Curiel, T.J.; De Cabo, R.; Franceschi, C.; Gems, D.; et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell 2015, 14, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Calixto, A. Life without Food and the Implications for Neurodegeneration. Adv. Genet. 2015, 92, 53–74. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Wei, M.; Fabrizio, P.; Hu, J.; Ge, H.; Cheng, C.; Li, L.; Longo, V.D. Life Span Extension by Calorie Restriction Depends on Rim15 and Transcription Factors Downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008, 4, e13. [Google Scholar] [CrossRef] [Green Version]
- Fabrizio, P.; Pozza, F.; Pletcher, S.D.; Gendron, C.M.; Longo, V.D. Regulation of Longevity and Stress Resistance by Sch9 in Yeast. Science 2001, 292, 288–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, E.L.; Brunet, A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 2009, 8, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Honjoh, S.; Yamamoto, T.; Uno, M.; Nishida, E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 2009, 457, 726–730. [Google Scholar] [CrossRef]
- Grandison, R.C.; Wong, R.; Bass, T.M.; Partridge, L.; Piper, M.D.W. Effect of a Standardised Dietary Restriction Protocol on Multiple Laboratory Strains of Drosophila melanogaster. PLoS ONE 2009, 4, e4067. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nat. Cell Biol. 2013, 493, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending Healthy Life Span--From Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Parrella, E.; Maxim, T.; Maialetti, F.; Zhang, L.; Wan, J.; Wei, M.; Cohen, P.; Fontana, L.; Longo, V.D. Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer’s disease mouse model. Aging Cell 2013, 12, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Shanmuganayagam, D.; Weindruch, R. Caloric Restriction and Aging: Studies in Mice and Monkeys. Toxicol. Pathol. 2009, 37, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Hu, J.; King, J.; Jay, G.; Campbell, T.C. Inhibition of hepatocellular carcinoma development in hepatitis B virus transfected mice by low dietary casein. Hepatol. 1997, 26, 1351–1354. [Google Scholar] [CrossRef]
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, R.; De Cabo, R.; Anderson, R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017, 8, 14063. [Google Scholar] [CrossRef] [PubMed]
- Zimin, A.V.; Cornish, A.S.; Maudhoo, M.D.; Gibbs, R.M.; Zhang, X.; Pandey, S.; Meehan, D.T.; Wipfler, K.; E Bosinger, S.; Johnson, Z.P.; et al. A new rhesus macaque assembly and annotation for next-generation sequencing analyses. Biol. Direct 2014, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Kemnitz, J.W.; Weindruch, R.; Roecker, E.B.; Crawford, K.; Kaufman, P.L.; Ershler, W.B. Dietary Restriction of Adult Male Rhesus Monkeys: Design, Methodology, and Preliminary Findings From the First Year of Study. J. Gerontol. 1993, 48, B17–B26. [Google Scholar] [CrossRef]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Walford, R.L.; Mock, D.; Verdery, R.; Maccallum, T. Calorie Restriction in Biosphere 2: Alterations in Physiologic, Hematologic, Hormonal, and Biochemical Parameters in Humans Restricted for a 2-Year Period. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2002, 57, B211–B224. [Google Scholar] [CrossRef]
- Dorling, J.L.; Van Vliet, S.; Huffman, K.M.; E Kraus, W.; Bhapkar, M.; Pieper, C.F.; Stewart, T.; Das, S.K.; Racette, S.B.; Roberts, S.B.; et al. Effects of caloric restriction on human physiological, psychological, and behavioral outcomes: Highlights from CALERIE phase 2. Nutr. Rev. 2021, 79, 98–113. [Google Scholar] [CrossRef]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 6659–6663. [Google Scholar] [CrossRef] [Green Version]
- Rizza, W.; Veronese, N.; Fontana, L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res. Rev. 2014, 13, 38–45. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Suh, Y.-A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nat. Cell Biol. 1999, 401, 79–82. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.-H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Tellejohan, R.; Maudsley, S.; et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free. Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Gensous, N.; Franceschi, C.; Santoro, A.; Milazzo, M.; Garagnani, P.; Bacalini, M.G. The Impact of Caloric Restriction on the Epigenetic Signatures of Aging. Int. J. Mol. Sci. 2019, 20, 2022. [Google Scholar] [CrossRef] [Green Version]
- Calvanese, V.; Lara, E.; Kahn, A.; Fraga, M.F. The role of epigenetics in aging and age-related diseases. Ageing Res. Rev. 2009, 8, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Stampfer, M.J.; Giovannucci, E.; Ma, J.; Pollak, M. Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and prostate cancer risk: Epidemiological studies. Growth Horm. IGF Res. 2000, 10, S32–S33. [Google Scholar] [CrossRef]
- Giovannucci, E.; Pollak, M.; Platz, E.A.; Willett, W.C.; Stampfer, M.J.; Majeed, N.; Colditz, G.A.; Speizer, F.E.; Hankinson, S.E. Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and the risk of colorectal adenoma and cancer in the Nurses’ Health Study. Growth Horm. IGF Res. 2000, 10, S30–S31. [Google Scholar] [CrossRef]
- Guevara-Aguirre, J.; Balasubramanian, P.; Guevara-Aguirre, M.; Wei, M.; Madia, F.; Cheng, C.-W.; Hwang, D.; Martin-Montalvo, A.; Saavedra, J.; Ingles, S.; et al. Growth Hormone Receptor Deficiency Is Associated with a Major Reduction in Pro-Aging Signaling, Cancer, and Diabetes in Humans. Sci. Transl. Med. 2011, 3, 70ra13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178, 1102–1114.e17. [Google Scholar] [CrossRef] [PubMed]
- Kani, A.H.; Alavian, S.M.; Esmaillzadeh, A.; Adibi, P.; Haghighatdoost, F.; Azadbakht, L. Effects of a Low-Calorie, Low-Carbohydrate Soy Containing Diet on Systemic Inflammation Among Patients with Nonalcoholic Fatty Liver Disease: A Parallel Randomized Clinical Trial. Horm. Metab. Res. 2017, 49, 687–692. [Google Scholar] [CrossRef]
- Poitou, C.; Dalmas, E.; Renovato, M.; Benhamo, V.; Hajduch, F.; Abdennour, M.; Kahn, J.-F.; Veyrie, N.; Rizkalla, S.; Fridman, W.-H.; et al. CD14dimCD16+and CD14+CD16+Monocytes in Obesity and During Weight Loss. Arter. Thromb. Vasc. Biol. 2011, 31, 2322–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, R.A.; Wegiel, J.; Kumar, A.; Yu, W.H.; Peterhoff, C.; Cataldo, A.; Cuervo, A.M. Extensive Involvement of Autophagy in Alzheimer Disease: An Immuno-Electron Microscopy Study. J. Neuropathol. Exp. Neurol. 2005, 64, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.H.; Cuervo, A.M.; Kumar, A.; Peterhoff, C.M.; Schmidt, S.D.; Lee, J.-H.; Mohan, P.S.; Mercken, M.; Farmery, M.R.; Tjernberg, L.O.; et al. Macroautophagy—a novel β-amyloid peptide-generating pathway activated in Alzheimer’s disease. J. Cell Biol. 2005, 171, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kondo, K.; Motoki, K.; Homma, H.; Okazawa, H. Fasting activates macroautophagy in neurons of Alzheimer’s disease mouse model but is insufficient to degrade amyloid-beta. Sci. Rep. 2015, 5, 12115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucci, P. Caloric restriction: Is mammalian life extension linked to p53? Aging 2012, 4, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Finkel, T. Key proteins and pathways that regulate lifespan. J. Biol. Chem. 2017, 292, 6452–6460. [Google Scholar] [CrossRef] [Green Version]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Hall, M.N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017, 36, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Saraswat, K.; Rizvi, S.I. Novel strategies for anti-aging drug discovery. Expert Opin. Drug Discov. 2017, 12, 955–966. [Google Scholar] [CrossRef]
- Tulsian, R.; Velingkaar, N.; Kondratov, R. Caloric restriction effects on liver mTOR signaling are time-of-day dependent. Aging 2018, 10, 1640–1648. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; De Roos, B.; Duthie, S.J.; Crosley, L.K.; Mayer, C.; Horgan, G.; Colquhoun, I.J.; Le Gall, G.; Huber, F.; Kremer, W.; et al. Metabolomics of prolonged fasting in humans reveals new catabolic markers. Metabolomics 2010, 7, 375–387. [Google Scholar] [CrossRef]
- Grajower, M.M.; Horne, B.D. Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 2019, 11, 873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martínez, M.E.; Villaseñor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, M.; Sedej, S. Cardiovascular benefits of intermittent fasting. Cardiovasc. Res. 2020, 116, e36–e38. [Google Scholar] [CrossRef] [PubMed]
- Eshghinia, S.; Mohammadzadeh, F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J. Diabetes Metab. Disord. 2013, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Stockman, M.-C.; Thomas, D.; Burke, J.; Apovian, C.M. Intermittent Fasting: Is the Wait Worth the Weight? Curr. Obes. Rep. 2018, 7, 172–185. [Google Scholar] [CrossRef]
- Parvaresh, A.; Razavi, R.; Abbasi, B.; Yaghoobloo, K.; Hassanzadeh, A.; Mohammadifard, N.; Safavi, S.M.; Hadi, A.; Clark, C.C. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: A randomized clinical trial. Complement. Ther. Med. 2019, 47, 102187. [Google Scholar] [CrossRef] [PubMed]
- De Toledo, F.W.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, e0209353. [Google Scholar] [CrossRef] [Green Version]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef] [Green Version]
- Finnell, J.S.; Saul, B.C.; Goldhamer, A.C.; Myers, T.R. Is fasting safe? A chart review of adverse events during medically supervised, water-only fasting. BMC Complement. Altern. Med. 2018, 18, 67. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, A.K. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Collier, R. Intermittent fasting: The science of going without. Can. Med. Assoc. J. 2013, 185, E363–E364. [Google Scholar] [CrossRef] [Green Version]
- Patterson, R.E.; Sears, D.D. Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.B.; Laub, D.R.; John, S. The effect on health of alternate day calorie restriction: Eating less and more than needed on alternate days prolongs life. Med. Hypotheses 2006, 67, 209–211. [Google Scholar] [CrossRef]
- Michalsen, A. Prolonged Fasting as a Method of Mood Enhancement in Chronic Pain Syndromes: A Review of Clinical Evidence and Mechanisms. Curr. Pain Headache Rep. 2010, 14, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [Green Version]
- García-Gaytán, A.C.; Miranda-Anaya, M.; Turrubiate, I.; Portugal, L.L.-D.; Bocanegra-Botello, G.N.; López-Islas, A.; Díaz-Muñoz, M.; Méndez, I. Synchronization of the circadian clock by time-restricted feeding with progressive increasing calorie intake. Resemblances and differences regarding a sustained hypocaloric restriction. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Antoni, R.; Robertson, T.M.; Robertson, M.D.; Johnston, J.D. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J. Nutr. Sci. 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Currenti, W.; Godos, J.; Castellano, S.; Mogavero, M.P.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Time restricted feeding and mental health: A review of possible mechanisms on affective and cognitive disorders. Int. J. Food Sci. Nutr. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Currenti, W.; Godos, J.; Castellano, S.; Caruso, G.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Association between Time Restricted Feeding and Cognitive Status in Older Italian Adults. Nutrients 2021, 13, 191. [Google Scholar] [CrossRef]
- Joslin, P.M.N.; Bell, R.K.; Swoap, S.J. Obese mice on a high-fat alternate-day fasting regimen lose weight and improve glucose tolerance. J. Anim. Physiol. Anim. Nutr. 2016, 101, 1036–1045. [Google Scholar] [CrossRef]
- Swoap, S.J.; Bingaman, M.J.; Hult, E.M.; Sandstrom, N.J. Alternate-day feeding leads to improved glucose regulation on fasting days without significant weight loss in genetically obese mice. Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R461–R469. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Yun, D.; Li, L.; Zhao, W.; Li, Y.; Liu, X.; Liu, Z. Alternate-day fasting alleviates diabetes-induced glycolipid metabolism disorders: Roles of FGF21 and bile acids. J. Nutr. Biochem. 2020, 83, 108403. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2019, 30, 462–476.e6. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Qin, Y.-L.; Shi, Z.-Y.; Chen, J.-H.; Zeng, M.-J.; Zhou, W.; Chen, R.-Q.; Chen, Z.-Y. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef]
- Varady, K.A.; Roohk, D.J.; Loe, Y.C.; McEvoy-Hein, B.K.; Hellerstein, M.K. Effects of modified alternate-day fasting regimens on adipocyte size, triglyceride metabolism, and plasma adiponectin levels in mice. J. Lipid Res. 2007, 48, 2212–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varady, K.A.; Hudak, C.S.; Hellerstein, M.K. Modified alternate-day fasting and cardioprotection: Relation to adipose tissue dynamics and dietary fat intake. Metabolism 2009, 58, 803–811. [Google Scholar] [CrossRef]
- A Varady, K.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroso, J.A.; Wasinski, F.; Donato, J. Prolonged fasting induces long-lasting metabolic consequences in mice. J. Nutr. Biochem. 2020, 84, 108457. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.L.; Kiersgaard, M.K.; Sørensen, D.B.; Mikkelsen, L.F. Fasting of mice: A review. Lab. Anim. 2013, 47, 225–240. [Google Scholar] [CrossRef]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2015, 70, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Das, S.K.; Pieper, C.F.; Lewis, M.R.; Klein, S.; Dixit, V.D.; Gupta, A.K.; Villareal, D.T.; Bhapkar, M.; Huang, M.; et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: A randomized controlled trial in non-obese humans. Aging 2016, 8, 1416–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thissen, J.-P.; Ketelslegers, J.-M.; Underwood, L.E. Nutritional Regulation of the Insulin-Like Growth Factors*. Endocr. Rev. 1994, 15, 80–101. [Google Scholar] [CrossRef]
- Villareal, D.T.; Fontana, L.; Das, S.K.; Redman, L.M.; Smith, S.R.; Saltzman, E.; Bales, C.W.; Rochon, J.; Pieper, C.F.; Huang, M.; et al. Effect of Two-Year Caloric Restriction on Bone Metabolism and Bone Mineral Density in Non-Obese Younger Adults: A Randomized Clinical Trial. J. Bone Miner. Res. 2016, 31, 40–51. [Google Scholar] [CrossRef]
- Di Renzo, L.; Rizzo, M.; Iacopino, L.; Sarlo, F.; Domino, E.; Jacoangeli, F.; Colica, C.; Sergi, D.; De Lorenzo, A. Body composition phenotype: Italian Mediterranean Diet and C677T MTHFR gene polymorphism interaction. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2555–2565. [Google Scholar]
- Tinsley, G.M.; Paoli, A. Time-restricted eating and age-related muscle loss. Aging 2019, 11, 8741–8742. [Google Scholar] [CrossRef]
- Romano, L.; Marchetti, M.; Gualtieri, P.; Renzo; Belcastro, M.; De Santis, G.L.; Perrone, M.A.; De Lorenzo, A.; Di Renzo, L.; De Santis, L.; et al. Effects of a Personalized VLCKD on Body Composition and Resting Energy Expenditure in the Reversal of Diabetes to Prevent Complications. Nutrients 2019, 11, 1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Donahoo, W.T. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef]
- De Amorim, A.C.R.; Costa, M.D.D.S.; Nunes, F.L.D.S.; Silva, M.D.G.B.D.; Leão, C.D.S.; Gadelha, P.C.F.P. Nutritional status and perioperative fasting time versus complications and hospital stay of surgical patients. Nutr. Hosp. 2015, 32, 878–887. [Google Scholar]
- Di Renzo, L.; Gratteri, S.; Sarlo, F.; Cabibbo, A.; Colica, C.; De Lorenzo, A. Individually Tailored Screening of Susceptibility to Sarcopenia Using p53 Codon 72 Polymorphism, Phenotypes, and Conventional Risk Factors. Dis. Mark. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Sarlo, F.; Petramala, L.; Iacopino, L.; Monteleone, G.; Colica, C.; De Lorenzo, A. Association between −308 G/A TNF-αPolymorphism and Appendicular Skeletal Muscle Mass Index as a Marker of Sarcopenia in Normal Weight Obese Syndrome. Dis. Mark. 2013, 35, 615–623. [Google Scholar] [CrossRef] [Green Version]
- De Lorenzo, A.; Andreoli, A.; Matthie, J.; Withers, P. Predicting body cell mass with bioimpedance by using theoretical methods: A technological review. J. Appl. Physiol. 1997, 82, 1542–1558. [Google Scholar] [CrossRef] [Green Version]
- Di Renzo, L.; Del Gobbo, V.; Bigioni, M.; Premrov, M.G.; Cianci, R.; De Lorenzo, A. Body Composition Analyses in Nor-mal Weight Obese Women. Eur. Rev. Med. Pharmacol. Sci. 2006, 10, 191–196. [Google Scholar] [PubMed]
- Haluzík, M.; Mráz, M. Intermittent Fasting and Prevention of Diabetic Retinopathy: Where Do We Go From Here? Diabetes 2018, 67, 1745–1747. [Google Scholar] [CrossRef] [Green Version]
- Kaye, W. Neurobiology of anorexia and bulimia nervosa. Physiol. Behav. 2008, 94, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Fortinguerra, F.; Ambrosino, F.; Pierantozzi, A.; DaCas, R.; Trotta, F.; Cangini, A. L’uso degli antibiotici in Italia. Il rapporto nazionale. OsMed 2019; Agenzia Italiana del Farmaco: Roma, Italy, 2021.
- Products, N.A.A. (Nda) E.P.O.D. Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 13. [Google Scholar] [CrossRef] [Green Version]
- Dulloo, A.; Seydoux, J.; Girardier, L. Potentiation of the thermogenic antiobesity effects of ephedrine by dietary methylxanthines: Adenosine antagonism or phosphodiesterase inhibition? Metabolism 1992, 41, 1233–1241. [Google Scholar] [CrossRef]
- Schubert, M.M.; Irwin, C.; Seay, R.F.; Clarke, H.E.; Allegro, D.; Desbrow, B. Caffeine, coffee, and appetite control: A review. Int. J. Food Sci. Nutr. 2017, 68, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Nehlig, A. Effects of coffee/caffeine on brain health and disease: What should I tell my patients? Pract. Neurol. 2016, 16, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Madeira, M.H.; Boia, R.; Ambrósio, A.F.; Santiago, A.R. Having a Coffee Break: The Impact of Caffeine Consumption on Microglia-Mediated Inflammation in Neurodegenerative Diseases. Mediat. Inflamm. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.; Beserra, B.; Reis, C.; Dorea, J.G.; Da Costa, T.; A Amato, A. Consumption of coffee or caffeine and serum concentration of inflammatory markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2017, 59, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, C. Alan Goldhamer, Dc: Water Fasting-The Clinical Effectiveness of Rebooting Your Body. Integr. Med. 2014, 13, 52–57. [Google Scholar]
- Di Renzo, L.; Cioccoloni, G.; Falco, S.; Abenavoli, L.; Moia, A.; Salimei, P.S.; De Lorenzo, A. Influence of FTO rs9939609 and Mediterranean diet on body composition and weight loss: A randomized clinical trial. J. Transl. Med. 2018, 16, 308. [Google Scholar] [CrossRef]
- Sands, W.A.; McNeal, J.R.; Murray, S.R.; Ramsey, M.W.; Sato, K.; Mizuguchi, S.; Stone, M.H. Stretching and Its Effects on Recovery. Strength Cond. J. 2013, 35, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Zouhal, H.; Saeidi, A.; Salhi, A.; Li, H.; Essop, M.F.; Laher, I.; Rhibi, F.; Amani-Shalamzari, S.; Ben Abderrahman, A. Exercise Training and Fasting: Current Insights. Open Access J. Sports Med. 2020, 11, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Madaniyazi, L.; Zhou, Y.; Li, S.; Williams, G.; Jaakkola, J.J.; Liang, X.; Liu, Y.; Wu, S.; Guo, Y. Outdoor Temperature, Heart Rate and Blood Pressure in Chinese Adults: Effect Modification by Individual Characteristics. Sci. Rep. 2016, 6, 21003. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-M.; Kim, S.; Cheong, H.-K.; Ahn, B.; Choi, K. Effects of Heat Wave on Body Temperature and Blood Pressure in the Poor and Elderly. Environ. Health Toxicol. 2012, 27, 2012013. [Google Scholar] [CrossRef]
- Zacny, J.P.; Wit, H. Effects of a 24-hour fast on cigarette smoking in humans. Br. J. Addict. 1990, 85, 555–560. [Google Scholar] [CrossRef] [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health the Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Reports of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014.
- Di Renzo, L.; Cinelli, G.; Dri, M.; Gualtieri, P.; Attinà, A.; Leggeri, C.; Cenname, G.; Esposito, E.; Pujia, A.; Chiricolo, G.; et al. Mediterranean Personalized Diet Combined with Physical Activity Therapy for the Prevention of Cardiovascular Diseases in Italian Women. Nutrients 2020, 12, 3456. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Avolio, E.; Bollero, P.; De Miranda, R.C.; Ferraro, S.; Salimei, P.S.; De Lorenzo, A.; Di Renzo, L. Evidences of a New Psychobiotic Formulation on Body Composition and Anxiety. Mediat. Inflamm. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Colica, C.; Merra, G.; Gasbarrini, A.; De Lorenzo, A.; Cioccoloni, G.; Gualtieri, P.; A Perrone, M.; Bernardini, S.; Bernardo, V.; Di Renzo, L.; et al. Efficacy and safety of very-low-calorie ketogenic diet: A double blind randomized crossover study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2274–2289. [Google Scholar]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Leggeri, C.; Cinelli, G.; Tarsitano, M.G.; Caparello, G.; Carrano, E.; et al. COVID-19: Is there a role for immunonutrition in obese patient? J. Transl. Med. 2020, 18, 415. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Johnson, E.C. Water Intake, Water Balance, and the Elusive Daily Water Requirement. Nutrients 2018, 10, 1928. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.E.; Bertram, C.D. Lymphatic System Flows. Annu. Rev. Fluid Mech. 2018, 50, 459–482. [Google Scholar] [CrossRef]
- Souto-Gallardo, M.D.L.C.; Gascón, M.B.; Cruz, A.J. Effect of weight loss on metabolic control in people with type 2 diabetes mellitus: Systematic review. Nutr. Hosp. 2012, 26, 1242–1249. [Google Scholar]
- Cheng, C.-W.; Villani, V.; Buono, R.; Wei, M.; Kumar, S.; Yilmaz, O.H.; Cohen, P.; Sneddon, J.B.; Perin, L.; Longo, V.D. Fasting-Mimicking Diet Promotes Ngn3-Driven β-Cell Regeneration to Reverse Diabetes. Cell 2017, 168, 775–788.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burg, E.L.V.D.; Schoonakker, M.P.; Van Peet, P.G.; Marle, M.E.V.D.A.-V.; Van Dijk, K.W.; Longo, V.D.; Lamb, H.J.; Numans, M.E.; Pijl, H. Fasting in diabetes treatment (FIT) trial: Study protocol for a randomised, controlled, assessor-blinded intervention trial on the effects of intermittent use of a fasting-mimicking diet in patients with type 2 diabetes. BMC Endocr. Disord. 2020, 20, 94. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L.; et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef]
| Time Restricted Feeding (TRF) | Intermittent Fasting (IF) | Prolonged Fasting (PF) | ||
|---|---|---|---|---|
| Alternate Day Fasting (ADF) | Modified Alternate Day Fasting (MADF) | |||
| Definition | This is an eating pattern in which the food intake is restricted to a time window of 8–12 h or less every day [1]. | This form of IF involves fasting every other day or on certain days of the week. Ad libitum caloric intake is followed on non-fasting days [66]. | This is a form of IF, similar to ADF, with a severe and specific caloric restriction on fasting days. During fasting days, the caloric intake consists of 15–25% of the dietary needs. Ad libitum diet is followed on non-fasting days [67]. | PF consists in fasting for an extended period, from 4 to 7 days. It has been less commonly studied for longer periods in humans [68]. |
| Characteristics | Limiting the eating duration may be an effective strategy to reduce the overall caloric intake. It does not necessarily have to involve caloric restriction [69]. | Starving one day, feasting the next. Only during the fasting days is a caloric restriction expected [66]. | Restriction days are non-consecutive and include only a small introduction of food. During non-fasting days the intake of food is at leisure [65]. | During consecutive fasting days usually only water is permitted [70]. |
| Commonly Practiced Method | 16/8: feeding window of 8 h/day in which it is allow to consume food and 16 h of fasting [71]. 12/12: feeding and fasting windows last the same. This is claimed to be the simplest type of TRF to improve health and to maintain weight [72]. | 5/2: This is the most common example of ADF. Calories are severely restricted for 2 days (preferably non-consecutive), and then normal eating occurs for the other 5 days in the week [73]. | 5/2: 15%–25% of Total Daily Energy expenditure (TDEE) is suggested during 2 non-consecutive fasting days a week. Ad libitum food intake for the resting 5 days [74]. | No commonly practices methods are defined. Periods of deliberate fasting with restrictions on intake of solid food are practiced [75]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attinà, A.; Leggeri, C.; Paroni, R.; Pivari, F.; Dei Cas, M.; Mingione, A.; Dri, M.; Marchetti, M.; Di Renzo, L. Fasting: How to Guide. Nutrients 2021, 13, 1570. https://doi.org/10.3390/nu13051570
Attinà A, Leggeri C, Paroni R, Pivari F, Dei Cas M, Mingione A, Dri M, Marchetti M, Di Renzo L. Fasting: How to Guide. Nutrients. 2021; 13(5):1570. https://doi.org/10.3390/nu13051570
Chicago/Turabian StyleAttinà, Alda, Claudia Leggeri, Rita Paroni, Francesca Pivari, Michele Dei Cas, Alessandra Mingione, Maria Dri, Marco Marchetti, and Laura Di Renzo. 2021. "Fasting: How to Guide" Nutrients 13, no. 5: 1570. https://doi.org/10.3390/nu13051570
APA StyleAttinà, A., Leggeri, C., Paroni, R., Pivari, F., Dei Cas, M., Mingione, A., Dri, M., Marchetti, M., & Di Renzo, L. (2021). Fasting: How to Guide. Nutrients, 13(5), 1570. https://doi.org/10.3390/nu13051570