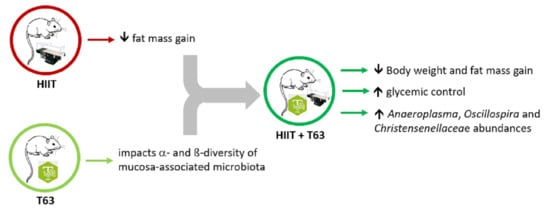
The TOTUM-63 Supplement and High-Intensity Interval Training Combination Limits Weight Gain, Improves Glycemic Control, and Influences the Composition of Gut Mucosa-Associated Bacteria in Rats on a High Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals Characteristics
2.2. Intervention
2.3. Food Consumption, Body Weight and Body Composition
2.4. Training Protocol
2.5. Fasting Glycemia, Fasting Insulinemia, and Oral Glucose Tolerance Test
2.6. Citrate Synthase Activity
2.7. Protein Extraction and Western Blotting
2.8. RNA Isolation, Reverse Transcription and Quantitative Real-Time PCR
2.9. Other Biochemical Analyses
2.10. Concentration of Fecal Short Chain Fatty Acids (SCFAs) by Gas Chromatography
2.11. Microbiota Composition Analysis by Illumina Sequencing
2.12. Statistical Analysis
3. Results
3.1. HFD for 16 Weeks Significantly Alters Body Composition in Wistar Rats
3.2. The T63-HIIT Combination Limits Body Weight and Fat Mass Gain, Independently of the Energy Intake
3.3. The HIIT-T63 Combination Improves Glycemic Control
3.4. The HIIT-T63 Combination Improves Muscle Oxidative Metabolism
3.5. T63 Supplementation and/or HIIT Enhances the Pro-/Anti-Oxidant Balance in Plasma
3.6. T63 and HIIT Differently Modulate the Intestinal Mucosa-Associated Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- ADA 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [CrossRef] [Green Version]
- Palacios, O.M.; Kramer, M.; Maki, K.C. Diet and Prevention of Type 2 Diabetes Mellitus: Beyond Weight Loss and Exercise. Expert Rev. Endocrinol. Metab. 2019, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A High-Risk State for Diabetes Development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- Tuso, P. Prediabetes and Lifestyle Modification: Time to Prevent a Preventable Disease. Perm. J. 2014, 18, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Gaggini, M.; DeFronzo, R.A. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results from the San Antonio Metabolism Study. Diabetes 2017, 66, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Apovian, C.M.; Okemah, J.; O’Neil, P.M. Body Weight Considerations in the Management of Type 2 Diabetes. Adv. Ther. 2019, 36, 44–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K.; American College of Sports Medicine American College of Sports Medicine Position Stand. Appropriate Physical Activity Intervention Strategies for Weight Loss and Prevention of Weight Regain for Adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-Intensity Interval Training in Patients with Lifestyle-Induced Cardiometabolic Disease: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Maillard, F.; Pereira, B.; Boisseau, N. Effect of High-Intensity Interval Training on Total, Abdominal and Visceral Fat Mass: A Meta-Analysis. Sports Med. 2018, 48, 269–288. [Google Scholar] [CrossRef]
- Dupuit, M.; Maillard, F.; Pereira, B.; Marquezi, M.L.; Lancha, A.H.; Boisseau, N. Effect of High Intensity Interval Training on Body Composition in Women before and after Menopause: A Meta-Analysis. Exp. Physiol. 2020, 105, 1470–1490. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Little, J.P. Effectiveness and Safety of High-Intensity Interval Training in Patients with Type 2 Diabetes. Diabetes Spectr. 2015, 28, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [Green Version]
- Wormgoor, S.G.; Dalleck, L.C.; Zinn, C.; Borotkanics, R.; Harris, N.K. High-Intensity Interval Training Is Equivalent to Moderate-Intensity Continuous Training for Short- and Medium-Term Outcomes of Glucose Control, Cardiometabolic Risk, and Microvascular Complication Markers in Men with Type 2 Diabetes. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological Adaptations to Low-Volume, High-Intensity Interval Training in Health and Disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Forbes, S.C.; Candow, D.G.; Smith-Ryan, A.E.; Hirsch, K.R.; Roberts, M.D.; VanDusseldorp, T.A.; Stratton, M.T.; Kaviani, M.; Little, J.P. Supplements and Nutritional Interventions to Augment High-Intensity Interval Training Physiological and Performance Adaptations-A Narrative Review. Nutrients 2020, 12, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavanelle, V.; Otero, Y.F.; Le Joubioux, F.; Ripoche, D.; Bargetto, M.; Vluggens, A.; Montaurier, C.; Pickering, G.; Ducheix, G.; Dubray, C.; et al. Effects of Totum-63 on Glucose Homeostasis and Post-Prandial Glycemia: A Translational Study. AJP-Endocrinol. Metabol. 2021. [Google Scholar] [CrossRef]
- Blandino, G.; Inturri, R.; Lazzara, F.; Di Rosa, M.; Malaguarnera, L. Impact of Gut Microbiota on Diabetes Mellitus. Diabetes Metab. 2016, 42, 303–315. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Assanelli, D.; Testa, C.; Romano, C.; Dioguardi, F.S.; Aquilani, R. Effects of Chronic Exercise on Gut Microbiota and Intestinal Barrier in Human with Type 2 Diabetes. Minerva Med. 2019, 110, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Wisniewski, P.J. Exercise Is a Novel Promoter of Intestinal Health and Microbial Diversity. Exerc. Sport Sci. Rev. 2017, 45, 41–47. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alp, P.R.; Newsholme, E.A.; Zammit, V.A. Activities of Citrate Synthase and NAD+-Linked and NADP+-Linked Isocitrate Dehydrogenase in Muscle from Vertebrates and Invertebrates. Biochem. J. 1976, 154, 689–700. [Google Scholar] [CrossRef]
- Stephenson, E.J.; Camera, D.M.; Jenkins, T.A.; Kosari, S.; Lee, J.S.; Hawley, J.A.; Stepto, N.K. Skeletal Muscle Respiratory Capacity Is Enhanced in Rats Consuming an Obesogenic Western Diet. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1541–E1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavanelle, V.; Boisseau, N.; Otero, Y.F.; Combaret, L.; Dardevet, D.; Montaurier, C.; Delcros, G.; Peltier, S.L.; Sirvent, P. Effects of High-Intensity Interval Training and Moderate-Intensity Continuous Training on Glycaemic Control and Skeletal Muscle Mitochondrial Function in Db/Db Mice. Sci. Rep. 2017, 7, 204. [Google Scholar] [CrossRef]
- Gilda, J.E.; Ghosh, R.; Cheah, J.X.; West, T.M.; Bodine, S.C.; Gomes, A.V. Western Blotting Inaccuracies with Unverified Antibodies: Need for a Western Blotting Minimal Reporting Standard (WBMRS). PLoS ONE 2015, 10, e0135392. [Google Scholar] [CrossRef] [PubMed]
- Groussard, C.; Maillard, F.; Vazeille, E.; Barnich, N.; Sirvent, P.; Otero, Y.F.; Combaret, L.; Madeuf, E.; Sourdrille, A.; Delcros, G.; et al. Tissue-Specific Oxidative Stress Modulation by Exercise: A Comparison between MICT and HIIT in an Obese Rat Model. Oxid. Med. Cell Longev. 2019, 2019, 1965364. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An Improved Greengenes Taxonomy with Explicit Ranks for Ecological and Evolutionary Analyses of Bacteria and Archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of Composition of Microbiomes: A Novel Method for Studying Microbial Composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and Exercise in the Prevention and Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Montefusco, L.; D’Addio, F.; Loretelli, C.; Ben Nasr, M.; Garziano, M.; Rossi, A.; Pastore, I.; Plebani, L.; Lunati, M.E.; Bolla, A.M.; et al. Anti-Inflammatory Effects of Diet and Caloric Restriction in Metabolic Syndrome. J. Endocrinol. Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- Maillard, F.; Rousset, S.; Pereira, B.; Boirie, Y.; Duclos, M.; Boisseau, N. High-Intensity Interval Training Is More Effective than Moderate-Intensity Continuous Training in Reducing Abdominal Fat Mass in Postmenopausal Women with Type 2 Diabetes: A Randomized Crossover Study. Diabetes Metab. 2018, 44, 516–517. [Google Scholar] [CrossRef]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Body Composition in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef]
- Maillard, F.; Vazeille, E.; Sauvanet, P.; Sirvent, P.; Combaret, L.; Sourdrille, A.; Chavanelle, V.; Bonnet, R.; Otero, Y.F.; Delcros, G.; et al. High Intensity Interval Training Promotes Total and Visceral Fat Mass Loss in Obese Zucker Rats without Modulating Gut Microbiota. PLoS ONE 2019, 14, e0214660. [Google Scholar] [CrossRef] [Green Version]
- Shanaki, M.; Khosravi, M.; Khoshdooni-Farahani, A.; Dadashi, A.; Heydari, M.F.; Delfan, M.; Jafary, H.; Gorgani-Firuzjaee, S. High-Intensity Interval Training Reversed High-Fat Diet-Induced M1-Macrophage Polarization in Rat Adipose Tissue via Inhibition of NOTCH Signaling. J. Inflamm. Res. 2020, 13, 165–174. [Google Scholar] [CrossRef] [Green Version]
- de Araujo, G.G.; Papoti, M.; dos Reis, I.G.M.; de Mello, M.A.R.; Gobatto, C.A. Short and Long Term Effects of High-Intensity Interval Training on Hormones, Metabolites, Antioxidant System, Glycogen Concentration, and Aerobic Performance Adaptations in Rats. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millman, J.; Okamoto, S.; Kimura, A.; Uema, T.; Higa, M.; Yonamine, M.; Namba, T.; Ogata, E.; Yamazaki, S.; Shimabukuro, M.; et al. Metabolically and Immunologically Beneficial Impact of Extra Virgin Olive and Flaxseed Oils on Composition of Gut Microbiota in Mice. Eur. J. Nutr. 2020, 59, 2411–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, E.; Doherty, R.M.O.; Murphy, E.F.; Wall, R.; Sullivan, O.O.; Nilaweera, K.; Fitzgerald, G.F.; Cotter, P.D.; Ross, R.P.; Stanton, C. Impact of Dietary Fatty Acids on Metabolic Activity and Host Intestinal Microbiota Composition in C57BL/6J Mice. Br. J. Nutr. 2014, 111, 1905–1917. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Romero, S.; Léniz, A.; Martínez-Maqueda, D.; Amézqueta, S.; Fernández-Quintela, A.; Hereu, M.; Torres, J.L.; Portillo, M.P.; Pérez-Jiménez, J. Inter-Individual Variability in Insulin Response after Grape Pomace Supplementation in Subjects at High Cardiometabolic Risk: Role of Microbiota and MiRNA. Mol. Nutr. Food Res. 2021, 65, 2000113. [Google Scholar] [CrossRef]
- Plissonneau, C.; Capel, F.; Chassaing, B.; Dupuit, M.; Maillard, F.; Wawrzyniak, I.; Combaret, L.; Dutheil, F.; Etienne, M.; Mairesse, G.; et al. High-Intensity Interval Training and α-Linolenic Acid Supplementation Improve DHA Conversion and Increase the Abundance of Gut Mucosa-Associated Oscillospira Bacteria. Nutrients 2021, 13, 788. [Google Scholar] [CrossRef]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Yasunaga, K.; Matsuo, N.; Katsuragi, Y.; Komikado, M.; Tokimitsu, I.; Wilder, D.; Jones, F.; et al. Green Tea Catechin Consumption Enhances Exercise-Induced Abdominal Fat Loss in Overweight and Obese Adults. J. Nutr. 2009, 139, 264–270. [Google Scholar] [CrossRef]
- Ghasemi, E.; Nayebifar, S. Benefits of 10 Weeks of High-Intensity Interval Training and Green Tea Supplementation on Cardiovascular Risk Factors and VO2max in Overweight Women. J. Res. Med. Sci. 2019, 24, 79. [Google Scholar] [CrossRef]
- Kwon, E.-Y.; Jung, U.J.; Park, T.; Yun, J.W.; Choi, M.-S. Luteolin Attenuates Hepatic Steatosis and Insulin Resistance Through the Interplay Between the Liver and Adipose Tissue in Mice with Diet-Induced Obesity. Diabetes 2015, 64, 1658–1669. [Google Scholar] [CrossRef] [Green Version]
- Ebaid, G.M.; Seiva, F.R.; Rocha, K.K.; Souza, G.A.; Novelli, E.L. Effects of Olive Oil and Its Minor Phenolic Constituents on Obesity-Induced Cardiac Metabolic Changes. Nutr. J. 2010, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Min, J.-S.; Chae, U.; Lee, J.Y.; Song, K.-S.; Lee, H.-S.; Lee, H.J.; Lee, S.-R.; Lee, D.-S. Anti-Inflammatory Effect of Oleuropein on Microglia through Regulation of Drp1-Dependent Mitochondrial Fission. J. Neuroimmunol. 2017, 306, 46–52. [Google Scholar] [CrossRef]
- Choi, W.H.; Son, H.J.; Jang, Y.J.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin Ameliorates the Obesity-Induced Skeletal Muscle Atrophy by Attenuating Mitochondrial Dysfunction in the Muscle of Obese Mice. Mol. Nutr. Food Res. 2017, 61, 1700218. [Google Scholar] [CrossRef]
- Khalafi, M.; Mohebbi, H.; Symonds, M.E.; Karimi, P.; Akbari, A.; Tabari, E.; Faridnia, M.; Moghaddami, K. The Impact of Moderate-Intensity Continuous or High-Intensity Interval Training on Adipogenesis and Browning of Subcutaneous Adipose Tissue in Obese Male Rats. Nutrients 2020, 12, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-S.; Holloszy, J.O.; Kim, K.; Koh, J.-H. Exercise Training-Induced PPARβ Increases PGC-1α Protein Stability and Improves Insulin-Induced Glucose Uptake in Rodent Muscles. Nutrients 2020, 12, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, V.N.; de Lima, M.P.; Motta-Santos, D.; Pesquero, J.L.; de Andrade, R.V.; de Almeida, J.A.; Araujo, R.C.; Campbell, C.S.G.; Lewis, J.E.; Simões, H.G. Role of Exercise Intensity on GLUT4 Content, Aerobic Fitness and Fasting Plasma Glucose in Type 2 Diabetic Mice. Cell Biochem. Funct. 2015, 33, 435–442. [Google Scholar] [CrossRef]
- Alkhateeb, H.; Al-Duais, M.; Qnais, E. Beneficial Effects of Oleuropein on Glucose Uptake and on Parameters Relevant to the Normal Homeostatic Mechanisms of Glucose Regulation in Rat Skeletal Muscle. Phytother. Res. 2018, 32, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Tsukahara, C.; Ikeda, N.; Sone, Y.; Ishikawa, T.; Ichi, I.; Koike, T.; Aoki, Y. Oleuropein Improves Insulin Resistance in Skeletal Muscle by Promoting the Translocation of GLUT4. J. Clin. Biochem. Nutr. 2017, 61, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Murotomi, K.; Umeno, A.; Yasunaga, M.; Shichiri, M.; Ishida, N.; Koike, T.; Matsuo, T.; Abe, H.; Yoshida, Y.; Nakajima, Y. Oleuropein-Rich Diet Attenuates Hyperglycemia and Impaired Glucose Tolerance in Type 2 Diabetes Model Mouse. J. Agric. Food Chem. 2015, 63, 6715–6722. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.R.; Jafari, M.; Haghshenas, R.; Ravasi, A. Impact of Eight Weeks Endurance Training on Biochemical Parameters and Obesity-Induced Oxidative Stress in High Fat Diet-Fed Rats. J. Exerc. Nutr. Biochem. 2016, 20, 29–35. [Google Scholar] [CrossRef]
- Ben Salem, M.; Ben Abdallah Kolsi, R.; Dhouibi, R.; Ksouda, K.; Charfi, S.; Yaich, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Jamoussi, K.; et al. Protective Effects of Cynara Scolymus Leaves Extract on Metabolic Disorders and Oxidative Stress in Alloxan-Diabetic Rats. BMC Complement Altern. Med. 2017, 17, 328. [Google Scholar] [CrossRef]
- Elosua, R.; Molina, L.; Fito, M.; Arquer, A.; Sanchez-Quesada, J.L.; Covas, M.I.; Ordoñez-Llanos, J.; Marrugat, J. Response of Oxidative Stress Biomarkers to a 16-Week Aerobic Physical Activity Program, and to Acute Physical Activity, in Healthy Young Men and Women. Atherosclerosis 2003, 167, 327–334. [Google Scholar] [CrossRef]
- Osawa, T. Protective Role of Dietary Polyphenols in Oxidative Stress. Mechan. Ageing Dev. 1999, 111, 133–139. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic Acid Improves Hepatic Insulin Resistance via Antioxidant, Hypolipidemic and Anti-Inflammatory Effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- D’Addio, F.; La Rosa, S.; Maestroni, A.; Jung, P.; Orsenigo, E.; Ben Nasr, M.; Tezza, S.; Bassi, R.; Finzi, G.; Marando, A.; et al. Circulating IGF-I and IGFBP3 Levels Control Human Colonic Stem Cell Function and Are Disrupted in Diabetic Enteropathy. Cell Stem Cell 2015, 17, 486–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.M.; Berg Miller, M.E.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and Forced Exercise Differentially Alters the Gut Microbiome in C57BL/6J Mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Zhou, J.; Zheng, Y.; Xu, Z.; Li, Y.; Zhou, S.; Zhang, C. Beneficial Effects of Polysaccharide-Rich Extracts from Apocynum Venetum Leaves on Hypoglycemic and Gut Microbiota in Type 2 Diabetic Mice. Biomed. Pharmacother. 2020, 127, 110182. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liao, J.; Fang, Y.; Deng, H.; Yin, H.; Shen, B.; Hu, M. Six-Week Exercise Training with Dietary Restriction Improves Central Hemodynamics Associated With Altered Gut Microbiota in Adolescents With Obesity. Front. Endocrinol. 2020, 11, 569085. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzat-Zadeh, Z.; Henning, S.M.; Yang, J.; Woo, S.L.; Lee, R.-P.; Huang, J.; Thames, G.; Gilbuena, I.; Tseng, C.-H.; Heber, D.; et al. California Strawberry Consumption Increased the Abundance of Gut Microorganisms Related to Lean Body Weight, Health and Longevity in Healthy Subjects. Nutr. Res. 2020, 85, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated Gut Microbiome Abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae Is Associated with Reduced Visceral Adipose Tissue and Healthier Metabolic Profile in Italian Elderly. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The Human Gut Bacteria Christensenellaceae Are Widespread, Heritable, and Associated with Health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Haro, C.; Montes-Borrego, M.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Tinahones, F.J.; Landa, B.B.; et al. Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J. Clin. Endocrinol. Metab. 2016, 101, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Hoshino, Y.; Hosokawa, M.; Takeyama, H.; Higuchi, M. Effects of Short-Term Endurance Exercise on Gut Microbiota in Elderly Men. Physiol. Rep. 2018, 6, e13935. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Stephens, N.A.; Sparks, L.M. Resistance to the Beneficial Effects of Exercise in Type 2 Diabetes: Are Some Individuals Programmed to Fail? J. Clin. Endocrinol. Metab. 2015, 100, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Solomon, T.P.J. Sources of Inter-Individual Variability in the Therapeutic Response of Blood Glucose Control to Exercise in Type 2 Diabetes: Going Beyond Exercise Dose. Front. Physiol. 2018, 9, 896. [Google Scholar] [CrossRef] [Green Version]
- Malin, S.K.; Stewart, N.R. Metformin May Contribute to Inter-Individual Variability for Glycemic Responses to Exercise. Front. Endocrinol. 2020, 11, 519. [Google Scholar] [CrossRef]
- Sparks, L.M. Exercise Training Response Heterogeneity: Physiological and Molecular Insights. Diabetologia 2017, 60, 2329–2336. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.; Del Bo’, C.; Martini, D.; Porrini, M.; Riso, P. A Review of Registered Clinical Trials on Dietary (Poly)Phenols: Past Efforts and Possible Future Directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef]
- Korpela, K.; Flint, H.J.; Johnstone, A.M.; Lappi, J.; Poutanen, K.; Dewulf, E.; Delzenne, N.; de Vos, W.M.; Salonen, A. Gut Microbiota Signatures Predict Host and Microbiota Responses to Dietary Interventions in Obese Individuals. PLoS ONE 2014, 9, e90702. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Compound Type | Extract Content (g/100 g Dry Weight) |
|---|---|
| Total polyphenols | 14.36 |
| Total anthocyanins | 0.81 |
| Monocaffeoylquinic acids | 1.18 |
| Chlorogenic acid | 0.85 |
| Other monocaffeoylquinic acids | 0.33 |
| Dicaffeoylquinic acids | 0.98 |
| Cynarin | 0.24 |
| Other dicaffeoylquinic acids | 0.74 |
| Caffeic acid | 0.01 |
| Oleuropein | 3.72 |
| Oleuropein isomers | 0.20 |
| Hydroxytyrosol | 0.04 |
| Luteolin | 0.01 |
| Luteolin-7-O-glucoside | 0.38 |
| Luteolin-7-O-glucuronide | 0.38 |
| Apigenin | 0.01 |
| Apigenin-7-O-glucoside | 0.01 |
| Apigenin-7-O-glucuronide | 0.25 |
| Apigenin-6-C-glucoside-8-C-arabinoside (shaftoside) | 0.06 |
| Apigenin-6,8-C-diglucoside (vicenin 2) | 0.06 |
| Eriodictyol | <0.01 |
| Eriodictyol-7-O-glucoside | 0.11 |
| Okanin-4-O-glucoside (marein) | 0.05 |
| Isookanin-7-O-glucoside (flavanomarein) | 0.05 |
| Maritimetin-6-O-glucoside (maritimein) | 0.08 |
| Saponins | |
| Chrysantellin A | 0.01 |
| Chrysantellin B | 0.27 |
| Alkaloids | |
| Piperine | 0.004 |
| Fibers | |
| Soluble fibers | 13.7 |
| Insoluble fibers | 3.3 |
| Genes | Forward Primer 5′ → 3′ | Reverse Primer 5′ → 3′ |
|---|---|---|
| Il1β | ATCTCACAGCAGCATCTCGA | TAGCAGGTCGTCATCATCCC |
| Il6 | CCACTGCCTTCCCTACTTCA | TTCTGACAGTGCATCATCGC |
| Il10 | AGAGAACCATGGCCCAGAAA | TGAGTGTCACGTAGGCTTCT |
| Tnfα | TCATCCGTTCTCTACCCAGC | TACTTCAGCGTCTCGTGTGT |
| Ppargc1α | AATGCAGCGGTCTTAGCACT | GTGTGAGGAGGGTCATCGTT |
| Nrf1 | TTCCTCAGCCTCCGTCTTCT | ACACACCTTGCACTCACACC |
| Gapdh | CATGCCATCACTGCCACTCA | GCGGCATGTCAGATCCACAA |
| HFD | T63 | HIIT | HIIT+T63 | p | η2 | |
|---|---|---|---|---|---|---|
| Acetate | 26.1 ± 4.3 | 22.5 ± 5.6 | 26.7 ± 7.1 | 23.5 ± 3.1 | 0.24 | 0.11 |
| Butyrate | 2.1 ± 1.0 | 2.1 ± 1.4 | 3.1 ± 1.9 | 1.6 ± 0.6 | 0.15 | 0.14 |
| Propionate | 2.4 ± 0.8 | 2.2 ± 0.9 | 2.8 ± 1.7 | 1.9 ± 0.3 | 0.30 | 0.09 |
| Total SCFAs | 30.8 ± 5.5 | 26.8 ± 7.3 | 32.5 ± 9.9 | 27.1 ± 3.7 | 0.21 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupuit, M.; Chavanelle, V.; Chassaing, B.; Perriere, F.; Etienne, M.; Plissonneau, C.; Boscaro, A.; Barnich, N.; Pialoux, V.; Maugard, T.; et al. The TOTUM-63 Supplement and High-Intensity Interval Training Combination Limits Weight Gain, Improves Glycemic Control, and Influences the Composition of Gut Mucosa-Associated Bacteria in Rats on a High Fat Diet. Nutrients 2021, 13, 1569. https://doi.org/10.3390/nu13051569
Dupuit M, Chavanelle V, Chassaing B, Perriere F, Etienne M, Plissonneau C, Boscaro A, Barnich N, Pialoux V, Maugard T, et al. The TOTUM-63 Supplement and High-Intensity Interval Training Combination Limits Weight Gain, Improves Glycemic Control, and Influences the Composition of Gut Mucosa-Associated Bacteria in Rats on a High Fat Diet. Nutrients. 2021; 13(5):1569. https://doi.org/10.3390/nu13051569
Chicago/Turabian StyleDupuit, Marine, Vivien Chavanelle, Benoit Chassaing, Fanny Perriere, Monique Etienne, Claire Plissonneau, Audrey Boscaro, Nicolas Barnich, Vincent Pialoux, Thierry Maugard, and et al. 2021. "The TOTUM-63 Supplement and High-Intensity Interval Training Combination Limits Weight Gain, Improves Glycemic Control, and Influences the Composition of Gut Mucosa-Associated Bacteria in Rats on a High Fat Diet" Nutrients 13, no. 5: 1569. https://doi.org/10.3390/nu13051569
APA StyleDupuit, M., Chavanelle, V., Chassaing, B., Perriere, F., Etienne, M., Plissonneau, C., Boscaro, A., Barnich, N., Pialoux, V., Maugard, T., Le Joubioux, F., Peltier, S., Sirvent, P., Otero, Y. F., & Boisseau, N. (2021). The TOTUM-63 Supplement and High-Intensity Interval Training Combination Limits Weight Gain, Improves Glycemic Control, and Influences the Composition of Gut Mucosa-Associated Bacteria in Rats on a High Fat Diet. Nutrients, 13(5), 1569. https://doi.org/10.3390/nu13051569