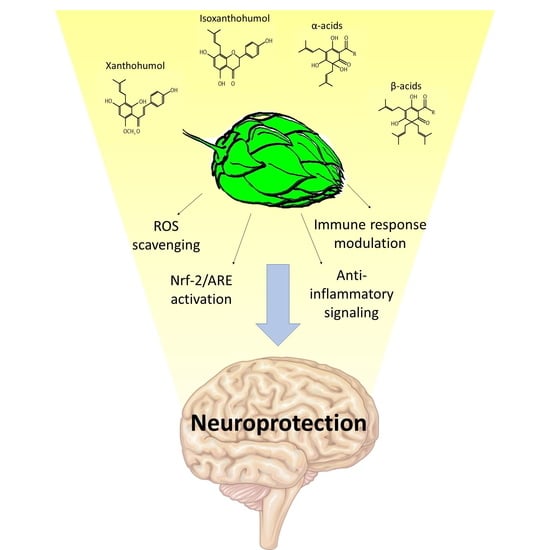
Redox and Anti-Inflammatory Properties from Hop Components in Beer-Related to Neuroprotection
Abstract
:1. Generalities
2. Redox Properties from Hop Derivatives on Beer
3. Anti-Inflammatory and Immunomodulatory Effects of Beer Compounds
Immunomodulatory Effect of Xanthohumol
4. Effect of Human Beer Consumption on Redox Environment and Immunomodulation
5. Hop Derivatives and Neuroprotection
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus Lupulus-A Story that Begs to Be Told. A Review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Osorio-Paz, I.; Brunauer, R.; Alavez, S. Beer and Its Non-Alcoholic Compounds in Health and Disease. Crit. Rev. Food Sci. Nutr. 2019, 60, 1–14. [Google Scholar] [CrossRef]
- Lasanta, C.; Durán-Guerrero, E.; Díaz, A.B.; Castro, R. Influence of Fermentation Temperature and Yeast Type on the Chemical and Sensory Profile of Handcrafted Beers. J. Sci. Food Agric. 2021, 101, 1174–1181. [Google Scholar] [CrossRef]
- Traversy, G.; Chaput, J.-P. Alcohol Consumption and Obesity: An Update. Curr. Obes. Rep. 2015, 4, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobak, M.; Skodova, Z.; Marmot, M. Beer and Obesity: A Cross-Sectional Study. Eur. J. Clin. Nutr. 2003, 57, 1250–1253. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Solà, J. Cardiovascular Risks and Benefits of Moderate and Heavy Alcohol Consumption. Nat. Rev. Cardiol. 2015, 12, 576–587. [Google Scholar] [CrossRef]
- de Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of Moderate Beer Consumption on Health and Disease: A Consensus Document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [Green Version]
- Romeo, J.; Wärnberg, J.; Nova, E.; Díaz, L.E.; González-Gross, M.; Marcos, A. Changes in the Immune System after Moderate Beer Consumption. Ann. Nutr. Metab. 2007, 51, 359–366. [Google Scholar] [CrossRef]
- Spaggiari, G.; Cignarelli, A.; Sansone, A.; Baldi, M.; Santi, D. To Beer or Not to Beer: A Meta-Analysis of the Effects of Beer Consumption on Cardiovascular Health. PLoS ONE 2020, 15, e0233619. [Google Scholar] [CrossRef]
- Rundio, A. Understanding Alcoholism. Nurs. Clin. North Am. 2013, 48, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Rontoyanni, V.G.; Protogerou, A.; Georgoulia, A.; Xenos, K.; Chrysou, J.; Sfikakis, P.P.; Sidossis, L.S. Acute Effects of Beer on Endothelial Function and Hemodynamics: A Single-Blind, Crossover Study in Healthy Volunteers. Nutrition 2013, 29, 1122–1126. [Google Scholar] [CrossRef] [Green Version]
- Gorinstein, S.; Zemser, M.; Berliner, M.; Goldstein, R.; Libman, I.; Trakhtenberg, S.; Caspi, A. Moderate Beer Consumption and Positive Biochemical Changes in Patients with Coronary Atherosclerosis. J. Intern. Med. 1997, 242, 219–224. [Google Scholar] [CrossRef]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inchingolo, F.; et al. Antioxidant Effect of Beer Polyphenols and Their Bioavailability in Dental-Derived Stem Cells (D-dSCs) and Human Intestinal Epithelial Lines (Caco-2) Cells. Stem Cells Int. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Oladokun, O.; Tarrega, A.; James, S.; Smart, K.; Hort, J.; Cook, D. The Impact of Hop Bitter Acid and Polyphenol Profiles on the Perceived Bitterness of Beer. Food Chem. 2016, 205, 212–220. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kocábek, T.; Nath, V.S.; Awasthi, P.; Shrestha, A.; Killi, U.K.; Jakse, J.; Patzak, J.; Krofta, K.; Matoušek, J. Dissection of Dynamic Transcriptome Landscape of Leaf, Bract, and Lupulin Gland in Hop (Humulus lupulus L.). Int. J. Mol. Sci. 2019, 21, 233. [Google Scholar] [CrossRef] [Green Version]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Porzel, A.; Schmidt, J.; Wessjohann, L.A. Metabolite Profiling and Fingerprinting of Commercial Cultivars of Humulus Lupulus L. (hop): A Comparison of MS and NMR Methods in Metabolomics. Metabolomics 2012, 8, 492–507. [Google Scholar] [CrossRef]
- Haseleu, G.; Lagemann, A.; Stephan, A.; Intelmann, D.; Dunkel, A.; Hofmann, T. Quantitative Sensomics Profiling of Hop-Derived Bitter Compounds Throughout a Full-Scale Beer Manufacturing Process. J. Agric. Food Chem. 2010, 58, 7930–7939. [Google Scholar] [CrossRef]
- Malowicki, M.G.; Shellhammer, T.H. Isomerization and Degradation Kinetics of Hop (Humulus lupulus) Acids in a Model Wort-Boiling System. J. Agric. Food Chem. 2005, 53, 4434–4439. [Google Scholar] [CrossRef]
- Aron, P.M.; Shellhammer, T.H. A Discussion of Polyphenols in Beer Physical and Flavour Stability. J. Inst. Brew. 2010, 116, 369–380. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated Flavonoids, Promising Nutraceuticals with Impressive Biological Activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative Analysis of Xanthohumol and Related Prenylflavonoids in Hops and Beer by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, E.J. Xanthohumol and Related Prenylflavonoids from Hops and Beer: To Your Good Health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Magalhaes, P.J.; Carvalho, D.O.; Cruz, J.M.; Guido, L.F.; Barros, A.A. Fundamentals and Health Benefits of Xan-Thohumol, a Natural Product Derived from Hops and Beer. Nat. Prod. Commun. 2009, 4, 591–610. [Google Scholar] [PubMed] [Green Version]
- Legette, L.; Karnpracha, C.; Reed, R.L.; Choi, J.; Bobe, G.; Christensen, J.M.; Rodriguez-Proteau, R.; Purnell, J.Q.; Stevens, J.F. Human Pharmacokinetics of Xanthohumol, an Antihyperglycemic Flavonoid from Hops. Mol. Nutr. Food Res. 2013, 58, 248–255. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid. Med. Cell. Longev. 2020, 2020, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA Damage and Reactive Oxygen Species in Neurodegenerative Disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Eckert, A. Brain Aging and Neurodegeneration: From a Mitochondrial Point of View. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [Green Version]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of phenolics content and antioxidant activity of different beer types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.L.; Outtrup, H.; Skibsted, L.H. Potential antioxidants in beer assessed by ESR spin trapping. J. Agric. Food Chem. 2000, 48, 3106–3111. [Google Scholar] [CrossRef]
- Spreng, S.; Hofmann, T. Activity-Guided Identification of In Vitro Antioxidants in Beer. J. Agric. Food Chem. 2018, 66, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Rivero, D.; Pérez-Magariño, S.; González-Sanjosé, M.L.; Valls-Belles, V.; Codoñer, A.P.; Muñiz, P. Inhibition of Induced DNA Oxidative Damage by Beers: Correlation with the Content of Polyphenols and Melanoidins. J. Agric. Food Chem. 2005, 53, 3637–3642. [Google Scholar] [CrossRef]
- Ottaviani, I.J.; Carrasquedo, F.; Keen, C.L.; Lazarus, A.S.; Schmitz, H.H.; Fraga, C.G. Influence of Flavan-3-Ols and Procyanidins on UVC-Mediated Formation of 8-oxo-7,8-dihydro-2′-Deoxyguanosine in Isolated DNA. Arch. Biochem. Biophys. 2002, 406, 203–208. [Google Scholar] [CrossRef]
- Gerhäuser, C. Phenolic Beer Compounds to Prevent Cancer. In Beer in Health and Disease Prevention; Elsevier BV: Amsterdam, The Netherlands, 2009; pp. 669–684. [Google Scholar]
- Tagasgira, M.; Watanabe, M.; Uemitsu, N. Antioxidative Activity of Hop Bitter Acids and Their Analogues. Biosci. Biotechnol. Biochem. 1995, 59, 740–742. [Google Scholar] [CrossRef]
- Yen, T.-L.; Hsu, C.-K.; Lu, W.-J.; Hsieh, C.-Y.; Hsiao, G.; Chou, D.-S.; Wu, G.-J.; Sheu, J.-R. Neuroprotective Effects of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus), in Ischemic Stroke of Rats. J. Agric. Food Chem. 2012, 60, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C.; Alt, A.; Heiss, E.; Gamal-Eldeen, A.; Klimo, K.; Knauft, J.; Neumann, I.; Scherf, H.-R.; Frank, N.; Bartsch, H.; et al. Cancer Chemopreventive Activity of Xanthohumol, a Natural Product Derived from Hop. Mol. Cancer Ther. 2002, 1, 959–969. [Google Scholar] [PubMed]
- Zhao, F.; Watanabe, Y.; Nozawa, H.; Daikonnya, A.; Kondo, K.; Kitanaka, S. Prenylflavonoids and Phloroglucinol Derivatives from Hops (Humulus Lupulus). J. Nat. Prod. 2005, 68, 43–49. [Google Scholar] [CrossRef]
- Zhao, F.; Nozawa, H.; Daikonnya, A.; Kondo, K.; Kitanaka, S. Inhibitors of Nitric Oxide Production from Hops (Humulus Lupulus L.). Biol. Pharm. Bull. 2003, 26, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Zhang, B.; Ge, C.; Peng, S.; Fang, J. Xanthohumol, a Polyphenol Chalcone Present in Hops, Activating Nrf2 Enzymes To Confer Protection against Oxidative Damage in PC12 Cells. J. Agric. Food Chem. 2015, 63, 1521–1531. [Google Scholar] [CrossRef]
- Miranda, C.L.; Stevens, J.F.; Ivanov, V.; McCall, M.; Frei, B.; Deinzer, M.L.; Buhler, D.R. Antioxidant and Prooxidant Actions of Prenylated and Nonprenylated Chalcones and Flavanones In Vitro. J. Agric. Food Chem. 2000, 48, 3876–3884. [Google Scholar] [CrossRef]
- Rodriguez, R.; Miranda, C.; Stevens, J.; Deinzer, M.; Buhler, D. Influence of Prenylated and Non-Prenylated Flavonoids on Liver Microsomal Lipid Peroxidation and Oxidative Injury in Rat Hepatocytes. Food Chem. Toxicol. 2001, 39, 437–445. [Google Scholar] [CrossRef]
- Lee, I.-S.; Lim, J.; Gal, J.; Kang, J.C.; Kim, H.J.; Kang, B.Y.; Choi, H.J. Anti-Inflammatory Activity of Xanthohumol Involves Heme Oxygenase-1 Induction via NRF2-ARE Signaling in Microglial BV2 Cells. Neurochem. Int. 2011, 58, 153–160. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, M.; Meseguer, I.; Sánchez-Reus, M.; Schultz, A.; Olivero, R.; Benedi, J.; Sánchez-Muniz, F. Beer Consumption Reduces Cerebral Oxidation Caused by Aluminum Toxicity by Normalizing Gene Expression of Tumor Necrotic Factor Alpha and Several Antioxidant Enzymes. Food Chem. Toxicol. 2008, 46, 1111–1118. [Google Scholar] [CrossRef]
- Valls-Belles, V.; Torres, C.; Muñiz, P.; Codoñer-Franch, P. Effect of Beer Consumption on Levels of Complex I and Complex IV Liver and Heart Mitochondrial Enzymes and Coenzymes Q9 and Q10 in Adriamycin-Treated Rats. Eur. J. Nutr. 2009, 49, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Diaz, L.E.; Cano, P.; Jimenez-Ortega, V.; Nova, E.; Romeo, J.; Marcos, A.; Esquifino, A.I. Effects of Moderate Consumption of Distilled and Fermented Alcohol on Some Aspects of Neuroimmunomodulation. Neuroimmunomodulation 2007, 14, 200–205. [Google Scholar] [CrossRef]
- Martinez, N.; Urpi-Sarda, M.; Martinez-Gonzalez, M.A.; Andres-Lacueva, C.; Mitjavila, M.T. Dealcoholised Beers Reduce Atherosclerosis and Expression of Adhesion Molecules in apoE-Deficient Mice. Br. J. Nutr. 2010, 105, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autelitano, D.J.; Howarth, E.A.; Pihl, E. Promoting Effect of Beer and Ethanol on Anti-Tumour Cytotoxicity: Unaffected Growth of a Transplantable Rat Tumour. Aust. J. Exp. Biol. Med Sci. 1984, 62, 507–514. [Google Scholar] [CrossRef]
- Wu, C.-N.; Sun, L.-C.; Chu, Y.-L.; Yu, R.-C.; Hsieh, C.-W.; Hsu, H.-Y.; Hsu, F.-C.; Cheng, K.-C. Bioactive Compounds with Anti-oxidative and Anti-Inflammatory Activities of Hop Extracts. Food Chem. 2020, 330, 127244. [Google Scholar] [CrossRef]
- Lupinacci, E.; Meijerink, J.; Vincken, J.-P.; Gabriele, B.; Gruppen, H.; Witkamp, R.F. Xanthohumol from Hop (Humulus Lupulus L.) Is an Efficient Inhibitor of Monocyte Chemoattractant Protein-1 and Tumor Necrosis Factor-α Release in LPS-Stimulated RAW 264.7 Mouse Macrophages and U937 Human Monocytes. J. Agric. Food Chem. 2009, 57, 7274–7281. [Google Scholar] [CrossRef]
- Schink, A.; Neumann, J.; Leifke, A.L.; Ziegler, K.; Fröhlich-Nowoisky, J.; Cremer, C.; Thines, E.; Weber, B.; Pöschl, U.; Schuppan, D.; et al. Screening of Herbal Extracts for TLR2-and TLR4-Dependent Anti-Inflammatory Effects. PLoS ONE 2018, 13, e0203907. [Google Scholar] [CrossRef] [PubMed]
- Hougee, S.; Faber, J.; Sanders, A.; Berg, W.B.V.D.; Garssen, J.; Smit, H.F.; Hoijer, M.A. Selective Inhibition of COX-2 by a Standardized CO2Extract of Humulus Lupulus in Vitroand Its Activity in a Mouse Model of Zymosan-Induced Arthritis. Planta Med. 2006, 72, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Fuchimoto, J.; Kojima, T.; Kobayashi, N.; Ohkuni, T.; Ogasawara, N.; Masaki, T.; Obata, K.; Nomura, K.; Kondoh, A.; Shigyo, T.; et al. Hop Water Extract Inhibits Double-stranded RNA-induced Thymic Stromal Lymphopoietin Release from Human Nasal Epithelial Cells. Am. J. Rhinol. Allergy 2012, 26, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ohya, R.; Kobayashi, K.; Ano, Y. Matured Hop Bitter Acids in Beer Improve Lipopolysaccharide-Induced Depression-Like Behavior. Front. Neurosci. 2019, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Saugspier, M.; Dorn, C.; Thasler, W.E.; Gehrig, M.; Heilmann, J.; Hellerbrand, C. Hop Bitter Acids Exhibit Anti-Fibrogenic Effects on Hepatic Stellate Cells In Vitro. Exp. Mol. Pathol. 2012, 92, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Van Cleemput, M.; Heyerick, A.; Libert, C.; Swerts, K.; Philippé, J.; De Keukeleire, D.; Haegeman, G.; De Bosscher, K. Hop Bitter Acids Efficiently Block Inflammation Independent of GRα, PPARα, or PPARγ. Mol. Nutr. Food Res. 2009, 53, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, N.; Li, X.; Chen, G.; Wang, C.; Lin, B.; Hou, Y. Characteristic α-Acid Derivatives from Humulus lupulus with Antineuroinflammatory Activities. J. Nat. Prod. 2017, 80, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Dohata, A.; Taniguchi, Y.; Hoshi, A.; Uchida, K.; Takashima, A.; Nakayama, H. Iso-α-acids, Bitter Components of Beer, Prevent Inflammation and Cognitive Decline Induced in a Mouse Model of Alzheimer’s Disease. J. Biol. Chem. 2017, 292, 3720–3728. [Google Scholar] [CrossRef] [Green Version]
- Ano, Y.; Takaichi, Y.; Uchida, K.; Kondo, K.; Nakayama, H.; Takashima, A. Iso-α-Acids, the Bitter Components of Beer, Suppress Microglial Inflammation in rTg4510 Tauopathy. Molecules 2018, 23, 3133. [Google Scholar] [CrossRef] [Green Version]
- Ano, Y.; Yoshikawa, M.; Takaichi, Y.; Michikawa, M.; Uchida, K.; Nakayama, H.; Takashima, A. Iso-α-Acids, Bitter Components in Beer, Suppress Inflammatory Responses and Attenuate Neural Hyperactivation in the Hippocampus. Front. Pharmacol. 2019, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Mahli, A.; Koch, A.; Fresse, K.; Schiergens, T.; Thasler, W.E.; Schönberger, C.; Bergheim, I.; Bosserhoff, A.; Hellerbrand, C. Iso-alpha Acids from Hops (Humulus Lupulus) Inhibit Hepatic Steatosis, Inflammation, and Fibrosis. Lab. Investig. 2018, 98, 1614–1626. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Ho, Y.-S.; Lai, C.-S.; Hsieh, S.-C.; Chen, L.-H.; Lin, E.; Ho, C.-T.; Pan, M.-H. Hexahydro-β-Acids Potently Inhibit 12-O-Tetradecanoylphorbol 13-Acetate-Induced Skin Inflammation and Tumor Promotion in Mice. J. Agric. Food Chem. 2013, 61, 11541–11549. [Google Scholar] [CrossRef]
- Cho, Y.-C.; Kim, H.J.; Kim, Y.-J.; Lee, K.Y.; Choi, H.J.; Lee, I.-S.; Kang, B.Y. Differential Anti-Inflammatory Pathway by Xanthohumol in IFN-γ and LPS-Activated Macrophages. Int. Immunopharmacol. 2008, 8, 567–573. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol Ameliorates Lipopolysaccharide (LPS)-Induced Acute Lung Injury via Induction of AMPK/GSK3β-Nrf2 Signal Axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Peluso, M.R.; Miranda, C.L.; Hobbs, D.J.; Proteau, R.R.; Stevens, J.F. Xanthohumol and Related Prenylated Flavonoids Inhibit Inflammatory Cytokine Production in LPS-Activated THP-1 Monocytes: Structure-Activity Relationships andIn SilicoBinding to Myeloid Differentiation Protein-2 (MD-2). Planta Med. 2010, 76, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-C.; You, S.-K.; Kim, H.J.; Cho, C.-W.; Lee, I.-S.; Kang, B.Y. Xanthohumol Inhibits IL-12 Production and Reduces Chronic Allergic Contact Dermatitis. Int. Immunopharmacol. 2010, 10, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Fu, W.; Chen, L.; Wang, Z.; Zhao, C.; Chen, G.; Liu, X.; Cai, Y.; Zhou, J.; Dai, Y.; et al. Determination of the Binding Mode for Anti-Inflammatory Natural Product Xanthohumol with Myeloid Differentiation Protein 2. Drug Des. Dev. Ther. 2016, ume 10, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Xuan, N.T.; Shumilina, E.; Gulbins, E.; Gu, S.; Götz, F.; Lang, F. Triggering of Dendritic Cell Apoptosis by Xanthohumol. Mol. Nutr. Food Res. 2010, 54, S214–S224. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deeb, D.; Liu, Y.; Gautam, S.; Dulchavsky, S.A.; Gautam, S.C. Immunomodulatory Activity of Xanthohumol: Inhibition of T Cell Proliferation, Cell-Mediated Cytotoxicity and Th1 Cytokine Production through Suppression of NF-κB. Immunopharmacol. Immunotoxicol. 2009, 31, 477–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Gao, X.; Deeb, R.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Anticancer Agent Xanthohumol Inhibits IL-2 Induced Signaling Pathways Involved in T Cell Proliferation. J. Exp. Ther. Oncol. 2012, 10, 1–8. [Google Scholar]
- Chen, X.; Li, Z.; Hong, H.; Wang, N.; Chen, J.; Lu, S.; Zhang, H.; Zhang, X.; Bei, C. Xanthohumol Suppresses Inflammation in Chondrocytes and Ameliorates Osteoarthritis in Mice. Biomed. Pharmacother. 2021, 137, 111238. [Google Scholar] [CrossRef]
- Cho, J.-M.; Yun, S.-M.; Choi, Y.-H.; Heo, J.; Kim, N.-J.; Kim, S.-H.; Kim, E.-H. Xanthohumol Prevents Dextran Sulfate Sodium-induced Colitis via Inhibition of IKKβ/NF-κB Signaling in Mice. Oncotarget 2017, 9, 866–880. [Google Scholar] [CrossRef] [Green Version]
- Dorn, C.; Kraus, B.; Motyl, M.; Weiss, T.S.; Gehrig, M.; Schölmerich, J.; Heilmann, J.; Hellerbrand, C. Xanthohumol, a Chalcon Derived from Hops, Inhibits Hepatic Inflammation and Fibrosis. Mol. Nutr. Food Res. 2010, 54, S205–S213. [Google Scholar] [CrossRef]
- Negrão, R.; Costa, R.; Duarte, D.; Gomes, T.T.; Mendanha, M.; Moura, L.; Vasques, L.; Azevedo, I.; Soares, R. Angiogenesis and Inflammation Signaling Are Targets of Beer Polyphenols on Vascular Cells. J. Cell. Biochem. 2010, 111, 1270–1279. [Google Scholar] [CrossRef]
- Dorn, C.; Heilmann, J.; Hellerbrand, C. Protective Effect of Xanthohumol on Toxin-Induced Liver Inflammation and Fibrosis. Int. J. Clin. Exp. Pathol. 2012, 5, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Dorn, C.; Massinger, S.; Wuzik, A.; Heilmann, J.; Hellerbrand, C. Xanthohumol Suppresses Inflammatory Response to Warm Ischemia–Reperfusion Induced Liver Injury. Exp. Mol. Pathol. 2013, 94, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Negrão, R.; Costa, R.; Duarte, D.; Gomes, T.T.; Coelho, P.; Guimarães, J.T.; Guardão, L.; Azevedo, I.; Soares, R. Xanthohumol-Supplemented Beer Modulates Angiogenesis and Inflammation in a Skin Wound Healing Model. Involvement of Local Adipocytes. J. Cell. Biochem. 2011, 113, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, M.; Fukizawa, S.; Nonaka, Y. Hop-Derived Prenylflavonoid Isoxanthohumol Suppresses Insulin Resistance by Changing the Intestinal Microbiota and Suppressing Chronic Inflammation in High Fat Diet-Fed Mice. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Pan, Y.; Gou, P.; Zhou, C.; Ma, L.; Liu, Q.; Du, Y.; Yang, J.; Wang, Q. Effect of Xanthohumol on Th1/Th2 Balance in a Breast Cancer Mouse Model. Oncol. Rep. 2017, 39, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoletti, T.; Fallarini, S.; Gugliesi, F.; Minassi, A.; Appendino, G.; Lombardi, G. Anti-Inflammatory and Vascularprotective Properties of 8-Prenylapigenin. Eur. J. Pharmacol. 2009, 620, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-L.; Chen, Y.-J.; Yu, J.; Du, Z.-Y.; Yuan, Q.; Sun, Y.-R.; Wu, X.; Li, Z.-Q.; Wu, X.-H.; Hu, J.; et al. ISO-Alpha-Acids Improve the Hematoma Resolution and Prevent Peri-Hematoma Inflammations by Transforming Microglia via PPARgamma-CD36 Axis in ICH Rats. Int. Immunopharmacol. 2020, 83, 106396. [Google Scholar] [CrossRef]
- Ano, Y.; Ohya, R.; Kondo, K.; Nakayama, H. Iso-α-acids, Hop-Derived Bitter Components of Beer, Attenuate Age-Related Inflammation and Cognitive Decline. Front. Aging Neurosci. 2019, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Bounds, W.; Betzing, K.W.; Stewart, R.M.; Holcombe, R.F. Social Drinking and the Immune Response: Impairment of Lymphokine-Activated Killer Activity. Am. J. Med Sci. 1994, 307, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Monobe, M.; Ando, K. Drinking Beer Reduces Radiation-Induced Chromosome Aberrations in Human Lymphocytes. J. Radiat. Res. 2002, 43, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imhof, A.; Blagieva, R.; Marx, N.; Koenig, W. Drinking Modulates Monocyte Migration in Healthy Subjects: A Randomised Intervention Study of Water, Ethanol, Red Wine and Beer with or without Alcohol. Diabetes Vasc. Dis. Res. 2008, 5, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Romeo, J.; Wärnberg, J.; Díaz, L.E.; González-Gross, M.; Marcos, A. Effects of Moderate Beer Consumption on First-Line Immunity of Healthy Adults. J. Physiol. Biochem. 2007, 63, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.R.M.; Bellés, V.V.; López-Jaén, A.B.; Marín, A.V.; Codoñer-Franch, P. Effects of Alcohol-Free Beer on Lipid Profile and Parameters of Oxidative Stress and Inflammation in Elderly Women. Nutrition 2009, 25, 182–187. [Google Scholar] [CrossRef]
- Scherr, J.; Nieman, D.C.; Schuster, T.; Habermann, J.; Rank, M.; Braun, S.; Pressler, A.; Wolfarth, B.; Halle, M. Nonalcoholic Beer Reduces Inflammation and Incidence of Respiratory Tract Illness. Med. Sci. Sports Exerc. 2012, 44, 18–26. [Google Scholar] [CrossRef]
- Daimiel, L.; Micó, V.; Díez-Ricote, L.; Ruiz-Valderrey, P.; Istas, G.; Rodríguez-Mateos, A.; Ordovás, J.M. Alcoholic and Non-Alcoholic Beer Modulate Plasma and Macrophage microRNAs Differently in a Pilot Intervention in Humans with Cardiovascular Risk. Nutrients 2020, 13, 69. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Magraner, E.; Condines, X.; Valderas-Martínez, P.; Roth, I.; Arranz, S.; Casas, R.; Navarro, M.; Hervas, A.; Sisó, A.; et al. Effects of Alcohol and Polyphenols from Beer on Atherosclerotic Biomarkers in High Cardiovascular Risk Men: A Randomized Feeding Trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 36–45. [Google Scholar] [CrossRef] [PubMed]
- López-Jaén, A.B.; Codoñer-Franch, P.; Martínez-Álvarez, J.R.; Villarino-Marín, A.; Valls-Bellés, V. Effect on Health of Non-Alcohol Beer and Hop Supplementation in a Group of Nuns in a Closed Order. Proc. Nutr. Soc. 2010, 69, 252. [Google Scholar] [CrossRef] [Green Version]
- Milivojevic, V.; Ansell, E.; Simpson, C.; Siedlarz, K.M.; Sinha, R.; Fox, H.C. Peripheral Immune System Adaptations and Motivation for Alcohol in Non-Dependent Problem Drinkers. Alcohol. Clin. Exp. Res. 2017, 41, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Zádori, D.; Veres, G.; Szalardy, L.; Klivenyi, P.; Vécsei, L. Alzheimer’s Disease: Recent Concepts on the Relation of Mitochondrial Disturbances, Excitotoxicity, Neuroinflammation, and Kynurenines. J. Alzheimer’s Dis. 2018, 62, 523–547. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.G. Concepts and Classification of Neurodegenerative Diseases. Handb. Clin. Neurol. 2018, 145, 301–307. [Google Scholar] [CrossRef]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy Metabolism and Inflammation in Brain Aging and Alzheimer’s Disease. Free. Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Jung, J.; Song, D.; Kräuter, M.; Kim, Y. Effects of Humulus Lupulus Extract on the Central Nervous System in Mice. Planta Med. 1993, 59, A691. [Google Scholar] [CrossRef]
- Schiller, H.; Forster, A.; Vonhoff, C.; Hegger, M.; Biller, A.; Winterhoff, H. Sedating Effects of Humulus Lupulus L. Extracts. Phytomedicine 2006, 13, 535–541. [Google Scholar] [CrossRef]
- Zanoli, P.; Rivasi, M.; Zavatti, M.; Brusiani, F.; Baraldi, M. New Insight in the Neuropharmacological Activity of Humulus Lupulus L. J. Ethnopharmacol. 2005, 102, 102–106. [Google Scholar] [CrossRef]
- Aoshima, H.; Takeda, K.; Okita, Y.; Hossain, S.J.; Koda, H.; Kiso, Y. Effects of Beer and Hop on Ionotropic γ-Aminobutyric Acid Receptors. J. Agric. Food Chem. 2006, 54, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, P.; Zavatti, M.; Rivasi, M.; Brusiani, F.; Losi, G.; Puia, G.; Avallone, R.; Baraldi, M. Evidence that the β-acids Fraction of Hops Reduces Central GABAergic Neurotransmission. J. Ethnopharmacol. 2007, 109, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Benkherouf, A.Y.; Soini, S.L.; Stompor, M.; Uusi-Oukari, M. Positive Allosteric Modulation of Native and Recombinant GABAA Receptors by Hops Prenylflavonoids. Eur. J. Pharmacol. 2019, 852, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lin, T.Y.; Lu, C.W.; Huang, S.K.; Wang, Y.C.; Wang, S.J. Xanthohumol-Induced Presynaptic Reduction of Glutamate Release in the Rat Hippocampus. Food Funct. 2016, 7, 212–226. [Google Scholar] [CrossRef]
- Wang, C.C.; Ho, Y.H.; Hung, C.F.; Kuo, J.R.; Wang, S.J. Xanthohumol, an Active Constituent from Hope, Affords Protection Against Kainic Acid-Induced Excitotoxicity in Rats. Neurochem. Int. 2020, 133, 104629. [Google Scholar] [CrossRef]
- Rancán, L.; Paredes, S.D.; García, I.; Muñoz, P.; García, C.; De Hontanar, G.L.; de la Fuente, M.; Vara, E.; Tresguerres, J.A.F. Protective Effect of Xanthohumol Against Age-Related Brain Damage. J. Nutr. Biochem. 2017, 49, 133–140. [Google Scholar] [CrossRef]
- Zamzow, D.R.; Elias, V.; Legette, L.L.; Choi, J.; Stevens, J.F.; Magnusson, K.R. Xanthohumol Improved Cognitive Flexibility in Young Mice. Behav. Brain Res. 2014, 275, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Oberbauer, E.; Urmann, C.; Steffenhagen, C.; Bieler, L.; Brunner, D.; Furtner, T.; Humpel, C.; Bäumer, B.; Bandtlow, C.; Couillard-Despres, S.; et al. Chroman-Like Cyclic Prenylflavonoids Promote Neuronal Differentiation and Neurite Outgrowth and are Neuroprotective. J. Nutr. Biochem. 2013, 24, 1953–1962. [Google Scholar] [CrossRef]
- Urmann, C.; Oberbauer, E.; Couillard-Despres, S.; Aigner, L.; Riepl, H. Neurodifferentiating Potential of 8-Prenylnaringenin and Related Compounds in Neural Precursor Cells and Correlation with Estrogen-Like Activity. Planta Med. 2015, 81, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Wang, J.; Chen, X.; Liu, P.; Wang, S.; Song, F.; Zhang, Z.; Zhu, F.; Liu, J.; Song, G.; et al. The Prenylflavonoid Xanthohumol Reduces Alzheimer-Like Changes and Modulates Multiple Pathogenic Molecular Pathways in the Neuro2a/APPswe Cell Model of AD. Front. Pharmacol. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ano, Y.; Hoshi, A.; Ayabe, T.; Ohya, R.; Uchida, S.; Yamada, K.; Kondo, K.; Kitaoka, S.; Furuyashiki, T. Iso-α-acids, the Bitter Components of Beer, Improve Hippocampus-Dependent Memory through Vagus Nerve Activation. FASEB J. 2019, 33, 4987–4995. [Google Scholar] [CrossRef] [Green Version]
- Ano, Y.; Kitaoka, S.; Ohya, R.; Kondo, K.; Furuyashiki, T. Hop Bitter Acids Increase Hippocampal Dopaminergic Activity in a Mouse Model of Social Defeat Stress. Int. J. Mol. Sci. 2020, 21, 9612. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Obara, K.; Saito, J.; Umeda, S.; Ano, Y. Effects of Hop Bitter Acids, Bitter Components in Beer, on Cognition in Healthy Adults: A Randomized Controlled Trial. J. Agric. Food Chem. 2019, 68, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ohnuma, T.; Obara, K.; Kondo, S.; Arai, H.; Ano, Y. Supplementation with Matured Hop Bitter Acids Improves Cognitive Performance and Mood State in Healthy Older Adults with Subjective Cognitive Decline. J. Alzheimer’s Dis. 2020, 76, 387–398. [Google Scholar] [CrossRef]



| Beer Compound | In Vitro/In Vivo/Clinical Study | Effect | References |
|---|---|---|---|
| Xanthohumol or Isoxanthohumol | In vitro | ||
| Monocytes/macrophages (0.5–20 µM). |
| [44,66,67,68,69,70] | |
| Dendritic cells (2–50 µM). |
| [71] | |
| T lymphocytes (1.25–40 µM). |
| [72,73] | |
| Mice primary chondrocytes. (10–50 µM). |
| [74] | |
| IEC-6 intestinal epithelium (25 µM). |
| [75] | |
| Primary hepatocytes and hepatic stellate cells (5–10 µM). |
| [75,76] | |
| HUVEC cells (0.001–10 µM). |
| [77] | |
| In vivo | |||
| Mice, dextran sodium sulfate-induced colitis (0.1–10 mg/kg orally). |
| [75] | |
| Liver inflammation, non-alcoholic steatohepatitis, CCl4-induced liver injury, ischemia/reperfusion-induced liver injury (0.5% w/w food). |
| [76,78,79] | |
| Oxalazone-induced inflammation (0.1–5% topically). |
| [69] | |
| LPS-induced lung injury (10–50 mg/kg intraperitoneally). |
| [67] | |
| Skin wound healing (10 mg/L beverage or 50 µM topically). |
| [77,80] | |
| High-fat diet-induced inflammation (0.01%). |
| [81] | |
| Mice, breast cancer (25–50 mg/kg gavage). |
| [82] | |
| 8-prenylnaringenin | In vitro | ||
| RAW 264.7 macrophages (1–30 µM). |
| [83] | |
| HUVEC cells (0.001–10 µM). |
| [77,83] | |
| Spleenic adherent cells (5 µg/mL). |
| [69] | |
| In vivo | |||
| Rat skin wound healing (50 µM topically) |
| [77] | |
| Hop iso-α-acids | In vitro | ||
| Primary hepatocytes and hepatic stellate cells (10–20 µg/mL). |
| [64] | |
| BV-2 microglial cells (1–100 µM). |
| [60] | |
| Mice primary microglia culture. (0.1–40 µM). |
| [61,63] | |
| In vivo | |||
| Western diet-induced non-alcoholic liver disease mice. (0.5% w/w in food) |
| [64] | |
| Rat intracerebral hemorrhage. (10 mg/kg intraperitoneally). |
| [84] | |
| 5xFAD mice (Alzheimer’s experimental model) (0.4–20 mg/kg orally). |
| [61,63] | |
| rTg4510 mice (tauopathy experimental model) (0.5% w/w in food). |
| [62] | |
| Aged mice. (0.5% w/w in food). |
| [85] | |
| Hop β-acids | In vivo | ||
| TPA-induced skin inflammation in mice (5–50 µg/mL topically). |
| [65] | |
| Hop bitter acids mix | In vitro | ||
| Hepatic stellate cells (10 µg/mL). |
| [58] | |
| L929sA fibroblasts (0–200 µM). |
| [59] | |
| In vivo | |||
| Vagotomized and LPS-intoxicated mice (1–50 mg/kg). |
| [57] | |
| Hop extract | In vitro | ||
| RAW 264.7 macrophages (0.1–100 µg/mL). |
| [52,53] | |
| THP-1 myeloid cells (0.1–2%). |
| [54] | |
| PBMCs (3.6–30 µg/mL). |
| [55] | |
| Human nasal epithelial cells (0.1–50 µg/mL). |
| [56] | |
| Beer | In vivo | ||
| 42 mL beer/kg body weight. |
| [50] | |
| Drinkable beer ad libitum. |
| [51] | |
| Clinical study on healthy subjects Short term (30 min–4 h after single ingestion). | |||
| Alcoholic beer (355–700 mL). |
| [86,87] | |
| Long term. (21–45 days of beer consumption). | |||
| Alcoholic beer (330 mL/day women, 660 mL/day men). |
| [8,88,89] | |
| Non-alcoholic beer (500–1500 mL daily). |
| [90,91] | |
| Clinical study on cardiovascular risk subjects. Long term. (14–28 days of beer consumption). | |||
| Alcoholic beer (500–660 mL/day men). |
| [92,93] | |
| Non-alcoholic beer (500–990 mL/day men). |
| [92,93] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazquez-Cervantes, G.I.; Ortega, D.R.; Blanco Ayala, T.; Pérez de la Cruz, V.; Esquivel, D.F.G.; Salazar, A.; Pineda, B. Redox and Anti-Inflammatory Properties from Hop Components in Beer-Related to Neuroprotection. Nutrients 2021, 13, 2000. https://doi.org/10.3390/nu13062000
Vazquez-Cervantes GI, Ortega DR, Blanco Ayala T, Pérez de la Cruz V, Esquivel DFG, Salazar A, Pineda B. Redox and Anti-Inflammatory Properties from Hop Components in Beer-Related to Neuroprotection. Nutrients. 2021; 13(6):2000. https://doi.org/10.3390/nu13062000
Chicago/Turabian StyleVazquez-Cervantes, Gustavo Ignacio, Daniela Ramírez Ortega, Tonali Blanco Ayala, Verónica Pérez de la Cruz, Dinora Fabiola González Esquivel, Aleli Salazar, and Benjamín Pineda. 2021. "Redox and Anti-Inflammatory Properties from Hop Components in Beer-Related to Neuroprotection" Nutrients 13, no. 6: 2000. https://doi.org/10.3390/nu13062000
APA StyleVazquez-Cervantes, G. I., Ortega, D. R., Blanco Ayala, T., Pérez de la Cruz, V., Esquivel, D. F. G., Salazar, A., & Pineda, B. (2021). Redox and Anti-Inflammatory Properties from Hop Components in Beer-Related to Neuroprotection. Nutrients, 13(6), 2000. https://doi.org/10.3390/nu13062000