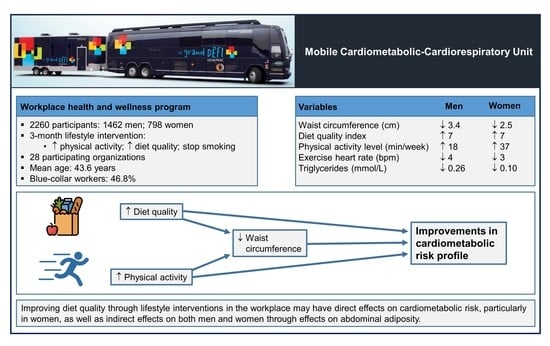
Targeting Diet Quality at the Workplace: Influence on Cardiometabolic Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Overall Diet Quality
2.3. Physical Activity Level
2.4. Anthropometric Measurements and Body Composition
2.5. Lipid Profile
2.6. Cardiorespiratory Fitness
2.7. Hypertriglyceridemic Waist Phenotype
2.8. Lifestyle Intervention
2.9. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases: Key Facts. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 February 2021).
- World Health Organization. Cardiovascular Diseases (CVDs): Key Facts. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 21 February 2021).
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Rundek, T.; Wright, C.B.; Anwar, Z.; Elkind, M.S.; Sacco, R.L. Ideal Cardiovascular Health Predicts Lower Risks of Myocardial Infarction, Stroke, and Vascular Death Across Whites, Blacks, and Hispanics: The northern Manhattan study. Circulation 2012, 125, 2975–2984. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen-Torvik, L.J.; Shay, C.M.; Abramson, J.G.; Friedrich, C.A.; Nettleton, J.A.; Prizment, A.E.; Folsom, A.R. Ideal cardiovascular health is inversely associated with incident cancer: The Atherosclerosis Risk in Communities study. Circulation 2013, 127, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younus, A.; Aneni, E.C.; Spatz, E.S.; Osondu, C.U.; Roberson, L.; Ogunmoroti, O.; Malik, R.; Ali, S.S.; Aziz, M.; Feldman, T.; et al. A Systematic Review of the Prevalence and Outcomes of Ideal Cardiovascular Health in US and Non-US Populations. Mayo Clin. Proc. 2016, 91, 649–670. [Google Scholar] [CrossRef] [Green Version]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2021 update: A report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Harrison, S.; Couillard, C.; Robitaille, J.; Vohl, M.C.; Bélanger, M.; Desroches, S.; Provencher, V.; Rabasa-Lhoret, R.; Bouchard, L.; Langlois, M.F.; et al. Assessment of the American Heart Association’s “Life’s simple 7” score in French-speaking adults from Quebec. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 684–691. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Waijers, P.M.C.M.; Feskens, E.; Ocké, M.C. A critical review of predefined diet quality scores. Br. J. Nutr. 2007, 97, 219–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Appel, L.J.; Van Horn, L. Components of a cardioprotective diet: New insights. Circulation 2011, 123, 2870–2891. [Google Scholar] [CrossRef] [PubMed]
- Dishman, R.K.; Oldenburg, B.; O’Neal, H.; Shephard, R.J. Worksite physical activity interventions. Am. J. Prev. Med. 1998, 15, 344–361. [Google Scholar] [CrossRef]
- Plotnikoff, R.C.; McCargar, L.J.; Wilson, P.M.; Loucaides, C.A. Efficacy of an E-Mail Intervention for the Promotion of Physical Activity and Nutrition Behavior in the Workplace Context. Am. J. Health Promot. 2005, 19, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Labour Force Survey. April 2019. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/190510/dq190510a-eng.htm (accessed on 22 February 2021).
- World Health Organization. Occupational Health. Available online: https://www.Who.Int/occupational_health/topics/workplace/en/ (accessed on 4 October 2020).
- Lévesque, V.; Vallières, M.; Poirier, P.; Després, J.-P.; Alméras, N. Targeting Abdominal Adiposity and Cardiorespiratory Fitness in the Workplace. Med. Sci. Sports Exerc. 2015, 47, 1342–1350. [Google Scholar] [CrossRef]
- Lévesque, V.; Poirier, P.; Després, J.-P.; Alméras, N. Relation Between a Simple Lifestyle Risk Score and Established Biological Risk Factors for Cardiovascular Disease. Am. J. Cardiol. 2017, 120, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Miller, P.E.; Mitchell, D.C.; Hartman, T.J.; Lawrence, F.R.; Sempos, C.T.; Smiciklas-Wright, H. Dietary screening tool identifies nutritional risk in older adults. Am. J. Clin. Nutr. 2009, 90, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buteau-Poulin, D.; Poirier, P.; Després, J.-P.; Alméras, N. Assessing nutritional quality as a ’vital sign’ of cardiometabolic health. Br. J. Nutr. 2019, 122, 195–205. [Google Scholar] [CrossRef]
- Günther, A.L.; Liese, A.D.; Bell, R.A.; Dabelea, D.; Lawrence, J.M.; Rodriguez, B.L.; Standiford, D.A.; Mayer-Davis, E.J. Association Between the Dietary Approaches to Hypertension Diet and Hypertension in Youth with Diabetes Mellitus. Hypertension 2009, 53, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Rumawas, M.E.; Dwyer, J.T.; McKeown, N.M.; Meigs, J.B.; Rogers, G.; Jacques, P.F. The Development of the Mediterranean-Style Dietary Pattern Score and Its Application to the American Diet in the Framingham Offspring Cohort. J. Nutr. 2009, 139, 1150–1156. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Jakes, R.; Bingham, S.; Welch, A.; Luben, R.; Day, N.; Wareham, N. Work and leisure time physical activity assessed using a simple, pragmatic, validated questionnaire and incident cardiovascular disease and all-cause mortality in men and women: The European Prospective Investigation into Cancer in Norfolk prospective population study. Int. J. Epidemiol. 2006, 35, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Gordon, C.C.; Chumlea, W.C.; Roche, A.F. Stature, Recumbent Length, and Weight. In Anthropometric Standardization Reference Manual; Lohman, T.G., Roche, A.F., Martorell, R., Eds.; Human Kinetics Books: Champaign, IL, USA, 1988; pp. 3–8. [Google Scholar]
- National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults-the evidence report. Obes. Res. 1998, 6, 51S–209S. [Google Scholar]
- Côté, C.-E.; Rhéaume, C.; Poirier, P.; Després, J.-P.; Alméras, N. Deteriorated Cardiometabolic Risk Profile in Individuals with Excessive Blood Pressure Response to Submaximal Exercise. Am. J. Hypertens. 2019, 32, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Wyndham, C.H. Submaximal tests for estimating maximum oxygen intake. Can. Med. Assoc. J. 1967, 96, 736–745. [Google Scholar] [PubMed]
- Astrand, P.O.; Ryhming, I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J. Appl. Physiol. 1954, 7, 218–221. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Riebe, D., Ehrman, J.K., Liguori, G., Magal, M., Eds.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Arsenault, B.J.; Lemieux, I.; Després, J.-P.; Wareham, N.J.; Kastelein, J.J.P.; Khaw, K.-T.; Boekholdt, S.M. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: Results from the EPIC-Norfolk Prospective Population Study. Can. Med. Assoc. J. 2010, 182, 1427–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemieux, I.; Pascot, A.; Couillard, C.; Lamarche, B.; Tchernof, A.; Alméras, N.; Bergeron, J.; Gaudet, D.; Tremblay, G.; Prud’homme, D.; et al. Hypertriglyceridemic waist. A marker of the atherogenic metabolic triad (hyperinsulinemia, hyperapolipoprotein B, small, dense LDL) in men? Circulation 2000, 102, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Brunner, E.; Domhof, S.; Langer, F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Rouseff, M.; Aneni, E.C.; Guzman, H.; Das, S.; Brown, D.; Osondu, C.U.; Spatz, E.; Shaffer, B.; Santiago-Charles, J.; Ochoa, T.; et al. One-year outcomes of an intense workplace cardio-metabolic risk reduction program among high-risk employees: The My Unlimited Potential. Obesity 2015, 24, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, D.R., Jr.; Tapsell, L.C. Food, not nutrients, is the fundamental unit in nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Ludwig, D.S. Dietary Guidelines in the 21st Century—A Time for Food. JAMA 2010, 304, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.; Inkeles, S.B. Effects of an intensive diet and exercise program on lipids in postmenopausal women. Women Health Issues 1999, 9, 155–161. [Google Scholar] [CrossRef]
- Groeneveld, I.F.; Proper, K.I.; Van Der Beek, A.J.; Hildebrandt, V.H.; Van Mechelen, W. Lifestyle-focused interventions at the workplace to reduce the risk of cardiovascular disease—A systematic review. Scand. J. Work. Environ. Health 2010, 36, 202–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couillard, C.; Després, J.-P.; Lamarche, B.; Bergeron, J.; Gagnon, J.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: Evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Petrogianni, M.; Kanellakis, S.; Kallianioti, K.; Argyropoulou, D.; Pitsavos, C.; Manios, Y. A multicomponent lifestyle intervention produces favourable changes in diet quality and cardiometabolic risk indices in hypercholesterolaemic adults. J. Hum. Nutr. Diet. 2013, 26, 596–605. [Google Scholar] [CrossRef]
- Remy, C.; Shubrook, J.H.; Nakazawa, M.; Drozek, D. Employer-Funded Complete Health Improvement Program: Preliminary Results of Biomarker Changes. J. Am. Osteopat. Assoc. 2017, 117, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Després, J.-P.; Alméras, N.; Gauvin, L. Worksite Health and Wellness Programs: Canadian Achievements & Prospects. Prog. Cardiovasc. Dis. 2014, 56, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, G.; Marangi, G.; Bontadi, D.; Fadda, E.; Cegolon, L.; Bortolotto, M.; Fedeli, U.; Marchiori, L. A worksite intervention to reduce the cardiovascular risk: Proposal of a study design easy to integrate within Italian organization of occupational health surveillance. BMC Public Health 2015, 15, 12. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.L.; Prince, S.A.; Elliott, C.G.; Mullen, K.A.; Tulloch, H.E.; Hiremath, S.; Cotie, L.M.; Pipe, A.L.; Reid, R.D. Impact of workplace physical activity interventions on physical activity and cardiometabolic health among working-age women: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003516. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Chandrasekaran, A.M.; Shivashankar, R.; Kondal, D.; Agrawal, A.; Panniyammakal, J.; Tandon, N.; Prabhakaran, D.; Sharma, M.; Goenka, S. Effect of workplace physical activity interventions on the cardio-metabolic health of working adults: Systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Monfort-Pires, M.; Folchetti, L.D.; Previdelli, A.N.; Siqueira-Catania, A.; De Barros, C.R.; Ferreira, S.R.G. Healthy Eating Index is associated with certain markers of inflammation and insulin resistance but not with lipid profile in individuals at cardiometabolic risk. Appl. Physiol. Nutr. Metab. 2014, 39, 497–502. [Google Scholar] [CrossRef]
- Gormley, S.E.; Swain, D.P.; High, R.; Spina, R.J.; Dowling, E.A.; Kotipalli, U.S.; Gandrakota, R. Effect of Intensity of Aerobic Training on VO2max. Med. Sci. Sports Exerc. 2008, 40, 1336–1343. [Google Scholar] [CrossRef]
- Helgerud, J.; Høydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic High-Intensity Intervals Improve VO2max More Than Moderate Training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, D.P.; Franklin, B.A. VO(2) reserve and the minimal intensity for improving cardiorespiratory fitness. Med. Sci. Sports Exerc. 2002, 34, 152–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, R.; de Lannoy, L.; Stotz, P.J. Separate Effects of Intensity and Amount of Exercise on Interindividual Cardiorespiratory Fitness Response. Mayo Clin. Proc. 2015, 90, 1506–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Prince, S.A.; Adamo, K.B.; Hamel, E.M.; Hardt, J.; Gorber, S.C.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56–64. [Google Scholar] [CrossRef] [Green Version]




| Variables | % |
|---|---|
| Sex | |
| Male | 64.7 |
| Female | 35.3 |
| Ethnicity | |
| Caucasian | 94.3 |
| Afro-Canadian | 1.3 |
| Latino-Canadian | 0.9 |
| Asian | 0.4 |
| First Nations | 0.3 |
| Others | 2.9 |
| Marital status | |
| Married/cohabiting | 74.8 |
| Unmarried | 18.1 |
| Separated/divorced | 6.6 |
| Widow/widower | 0.5 |
| Employee categories | |
| White collar | 39.7 |
| Blue collar | 46.8 |
| Unknown | 13.5 |
| Household income | |
| Low (<CAN 50,000) | 26.6 |
| Medium (CAN 50,000–80,000) | 14.1 |
| High (>CAN 80,000) | 54.2 |
| Unknown | 5.2 |
| Education | |
| <High school | 3.9 |
| High school | 36.9 |
| College | 29.3 |
| University | 19.9 |
| Post-graduate | 10.0 |
| Baseline | 3 Months | Δ | |
|---|---|---|---|
| Men | |||
| Anthropometric measurements and body composition (n) | 1430–1462 | 1459–1462 | 1429–1462 |
| Body mass index (kg/m2) | 27.4 (24.9, 30.4) | 26.8 (24.3, 29.4) | −0.5 (−1.1, 0.0) * |
| Waist circumference (cm) | 97.0 (89.3, 104.9) | 92.9 (85.9, 100.6) | −3.4 (−5.8, −1.2) * |
| Fat mass (kg) | 20.4 (15.3, 26.8) | 18.6 (14.1, 24.5) | −1.3 (−3.1, −0.1) * |
| Body fat (%) | 24.4 (20.1, 28.7) | 22.8 (18.9, 26.8) | −1.2 (−2.8, 0.0) * |
| Lifestyle habits (n) | 1462 | 1452–1462 | 1452–1462 |
| Diet quality index | 61 (52, 70) | 69 (60, 76) | 7 (1, 14) * |
| Physical activity level (min/week) | 210 (84, 378) | 222 (131, 339) | 18 (−108, 121) * |
| Cardiorespiratory profile (n) | 1384–1441 | 684–708 | 669–702 |
| Exercise heart rate (bpm) | 112 (103, 121) | 108 (100, 115) | −4 (−10, 2) * |
| Estimated VO2max (mL/min/kg) | 41.5 (35.5, 48.3) | 43.2 (36.7, 51.5) | 1.9 (−3.0, 7.2) * |
| Women | |||
| Anthropometric measurements and body composition (n) | 793–798 | 798 | 793–798 |
| Body mass index (kg/m2) | 25.0 (22.1, 28.9) | 24.5 (21.7, 28.4) | −0.4 (−0.9, 0.1) * |
| Waist circumference (cm) | 85.3 (77.1, 96.8) | 82.8 (75.2, 93.4) | −2.5 (−5.1, −0.1) * |
| Fat mass (kg) | 21.7 (15.9, 30.0) | 20.8 (14.6, 28.3) | −1.1 (−2.5, 0.1) * |
| Body fat (%) | 33.0 (27.2, 39.5) | 31.7 (25.7, 38.1) | −1.2 (−2.5, 0.1) * |
| Lifestyle habits (n) | 798 | 798 | 798 |
| Diet quality index | 65 (57, 72) | 72 (65, 79) | 7 (1, 13) * |
| Physical activity level (min/week) | 168 (84, 294) | 211 (132, 314) | 37 (−61, 126) * |
| Cardiorespiratory profile (n) | 725–776 | 170–176 | 164–173 |
| Exercise heart rate (bpm) | 125 (114, 135) | 120 (109, 131) | −3 (−9, 5) ‡ |
| Estimated VO2max (mL/min/kg) | 33.0 (27.7, 39.8) | 34.1 (28.9, 40.5) | 1.0 (−2.7, 5.1) § |
| Baseline | 3 Months | Δ | |
|---|---|---|---|
| Men | |||
| Lipid variables (n) | 1418–1462 | 721–728 | 712–728 |
| Total cholesterol (mmol/L) | 4.70 (4.10, 5.30) | 4.40 (3.90, 5.00) | −0.20 (−0.50, 0.10) * |
| LDL cholesterol (mmol/L) | 2.50 (2.00, 3.00) | 2.40 (2.00, 2.80) | −0.10 (−0.40, 0.20) * |
| HDL cholesterol (mmol/L) | 1.25 (1.07, 1.47) | 1.25 (1.07, 1.45) | 0.03 (−0.07, 0.13) * |
| Non-HDL cholesterol (mmol/L) | 3.40 (2.85, 3.95) | 3.16 (2.64, 3.65) | −0.20 (−0.50, 0.09) * |
| Cholesterol/HDL cholesterol | 3.70 (3.10, 4.30) | 3.50 (3.00, 4.20) | −0.20 (−0.56, 0.10) * |
| Triglycerides (mmol/L) | 1.81 (1.23, 2.72) | 1.44 (1.00, 2.26) | −0.26 (−0.85, 0.22) * |
| Women | |||
| Lipid variables (n) | 780–794 | 180–182 | 179–182 |
| Total cholesterol (mmol/L) | 4.60 (4.10, 5.10) | 4.40 (4.00, 5.10) | −0.10 (−0.40, 0.20) † |
| LDL cholesterol (mmol/L) | 2.40 (2.00, 2.80) | 2.40 (2.00, 2.80) | 0.00 (−0.30, 0.20) ‡ |
| HDL cholesterol (mmol/L) | 1.60 (1.34, 1.80) | 1.44 (1.28, 1.68) | −0.02 (−0.15, 0.10) † |
| Non-HDL cholesterol (mmol/L) | 2.97 (2.48, 3.51) | 2.94 (2.48, 3.41) | −0.12 (−0.34, 0.13) ‡ |
| Cholesterol/HDL cholesterol | 2.90 (2.50, 3.40) | 3.10 (2.60, 3.50) | −0.10 (−0.30, 0.10) |
| Triglycerides (mmol/L) | 1.18 (0.87, 1.69) | 1.05 (0.79, 1.55) | −0.10 (−0.40, 0.14) * |
| Total R2 × 100 | Partial R2 × 100 ∆ DQ Index | Partial R2 × 100 ∆ PAL | Partial R2 × 100 ∆ Exercise HR | |
|---|---|---|---|---|
| Men | ||||
| Anthropometric measurements and body composition (n = 669–697) | ||||
| ∆ Body mass index | 15.7 | 10.5 * | 1.5 † | 3.6 * |
| ∆ Waist circumference | 13.8 | 10.7 * | 1.8 † | 1.3 ‡ |
| ∆ Fat mass | 13.7 | 11.0 * | 1.2 ‡ | 1.5 † |
| ∆ Body fat | 9.6 | 8.3 * | 0.9 ‡ | 0.5 |
| Lipid profile (n = 678–694) | ||||
| ∆ Total cholesterol | 5.8 | 4.1 * | 1.7 † | – |
| ∆ LDL cholesterol | 0.4 | 0.4 | – | – |
| ∆ HDL cholesterol | 1.8 | 0.4 | – | 1.5 ‡ |
| ∆ Non-HDL cholesterol | 5.8 | 3.8 * | 2.0 † | – |
| ∆ Cholesterol/HDL cholesterol | 4.4 | 0.9 ‡ | 2.4 * | 1.1 ‡ |
| ∆ Triglycerides | 5.9 | 3.2 * | 1.3 ‡ | 1.5 ‡ |
| Women | ||||
| Anthropometric measurements and body composition (n = 168–173) | ||||
| ∆ Body mass index | 9.7 | 6.4 † | 3.3 ‡ | – |
| ∆ Waist circumference | 9.5 | 5.9 ‡ | 3.5 ‡ | – |
| ∆ Fat mass | 14.5 | 3.0 ‡ | 11.5 * | – |
| ∆ Body fat | 11.5 | – | 11.5 * | – |
| Lipid profile (n = 170–173) | ||||
| ∆ Total cholesterol | 14.0 | 4.6 ‡ | 9.4 * | – |
| ∆ LDL cholesterol | 2.4 | – | 2.4 ‡ | – |
| ∆ HDL cholesterol | 4.3 | – | 4.3 ‡ | – |
| ∆ Non-HDL cholesterol | 12.9 | 4.6 ‡ | 8.3 † | – |
| ∆ Cholesterol/HDL cholesterol | – | – | – | – |
| ∆ Triglycerides | 9.2 | 7.9 † | 1.3 | – |
| Total R2 × 100 | Partial R2 × 100 ∆ DQ Index | Partial R2 × 100 ∆ PAL | Partial R2 × 100 ∆ Exercise HR | Partial R2 × 100 ∆ Waist | |
|---|---|---|---|---|---|
| Men | |||||
| Lipid profile (n = 678–694) | |||||
| ∆ Total cholesterol | 9.8 | 1.4 ‡ | 1.0 ‡ | – | 7.3 * |
| ∆ LDL cholesterol | 0.8 | – | – | 0.3 | 0.5 |
| ∆ HDL cholesterol | 1.8 | 0.4 | – | 1.5 ‡ | – |
| ∆ Non-HDL cholesterol | 12.4 | 0.6 ‡ | 1.3 ‡ | – | 10.5 * |
| ∆ Cholesterol/HDL cholesterol | 9.9 | – | 1.0 ‡ | 0.5 | 8.4 * |
| ∆ Triglycerides | 11.4 | 0.5 | 0.8 ‡ | 0.8 ‡ | 9.4 * |
| Women | |||||
| Lipid profile (n = 170–173) | |||||
| ∆ Total cholesterol | 17.1 | 3.1 ‡ | 9.4 * | – | 4.6 ‡ |
| ∆ LDL cholesterol | 2.4 | – | 2.4 ‡ | – | – |
| ∆ HDL cholesterol | 4.3 | – | 4.3 ‡ | – | – |
| ∆ Non-HDL cholesterol | 16.1 | 3.0 ‡ | 8.3 † | – | 4.8 ‡ |
| ∆ Cholesterol/HDL cholesterol | – | – | – | – | – |
| ∆ Triglycerides | 13.4 | 4.6 ‡ | – | – | 8.9 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amil, S.; Lemieux, I.; Poirier, P.; Lamarche, B.; Després, J.-P.; Alméras, N. Targeting Diet Quality at the Workplace: Influence on Cardiometabolic Risk. Nutrients 2021, 13, 2283. https://doi.org/10.3390/nu13072283
Amil S, Lemieux I, Poirier P, Lamarche B, Després J-P, Alméras N. Targeting Diet Quality at the Workplace: Influence on Cardiometabolic Risk. Nutrients. 2021; 13(7):2283. https://doi.org/10.3390/nu13072283
Chicago/Turabian StyleAmil, Samira, Isabelle Lemieux, Paul Poirier, Benoît Lamarche, Jean-Pierre Després, and Natalie Alméras. 2021. "Targeting Diet Quality at the Workplace: Influence on Cardiometabolic Risk" Nutrients 13, no. 7: 2283. https://doi.org/10.3390/nu13072283
APA StyleAmil, S., Lemieux, I., Poirier, P., Lamarche, B., Després, J. -P., & Alméras, N. (2021). Targeting Diet Quality at the Workplace: Influence on Cardiometabolic Risk. Nutrients, 13(7), 2283. https://doi.org/10.3390/nu13072283