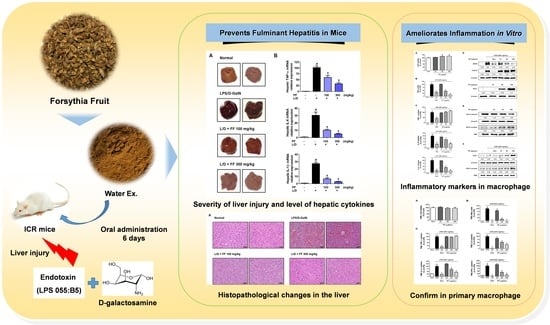
Forsythia Fruit Prevents Fulminant Hepatitis in Mice and Ameliorates Inflammation in Murine Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Materials and Reagents
2.3. Experimental Animals
2.4. Fulminant Hepatitis Mice Model by LPS/D-GalN Injection
2.5. RNA Extraction, DNA Synthesis, and Real-Time Reverse Transcription-Polymerase Chain Reaction
2.6. Histopathological Analysis
2.7. Preparation of Protein Extracts and Western Blot Analysis
2.8. Culture of Macophage Cell Line
2.9. Isolation and Culture of Mouse Peritoneal Macrophages
2.10. Cell Viability Assays
2.11. Measurement of NO and Inflammatory Cytokine Secretion
2.12. HPLC Instrument
2.13. Preperation of Standard and Sample Solutions
2.14. HPLC Analysis Method
2.15. Statistical Analysis
3. Results
3.1. Content of Major Compounds of FF
3.2. Regulatory Effects of FF on Serum Cytokine and Aminotransferase Levels in LPS/D-GalN-Induced Hepatitis in Mice
3.3. FF Protects Mice from Liver Injury and Regulates the Expression of Hepatic Cytokine mRNAs upon LPS/D-GalN Stimulation
3.4. Hepatoprotective Effects of FF on Histopathological Changes and Regulatory Effects on the Inflammatioy Proteins Expression
3.5. Regulatory Effects of FF on the Secretion of Inflammatory Mediators and Activation of Inflammatory/Antioxidant Pathways in LPS-Stimulated RAW 264.7 Macrophages
3.6. Inhibitory Effects of FF on LPS-Induced Inflammatory Mediator Levels in Primary Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernuau, J.; Rueff, B.; Benhamou, J.P. Fulminant and subfulminant liver failure: Definitions and causes. Semin. Liver Dis. 1986, 6, 97–106. [Google Scholar] [CrossRef]
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute liver failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef]
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef]
- Ng, K.K.; Lo, C.M. Liver transplantation in Asia: Past, present and future. Ann. Acad. Med. Singapore 2009, 38, 310–322. [Google Scholar]
- Soares, J.B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef] [Green Version]
- Farber, J.L.; Gill, G.; Konishi, Y. Prevention of galactosamine-induced liver necrosis by uridine. Am. J. Pathol. 1973, 72, 53–62. [Google Scholar] [PubMed]
- Yu, K.H.; Lee, S.Y.; Yang, H.M.; Ham, H.A.; Lee, S.U.; Chae, S.W.; Lee, Y.J. Effect of fermented water extracts from Ligularia fischeri on hepatotoxicity induced by D-galactosamine in rats. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1422–1430. [Google Scholar] [CrossRef]
- Chojkier, M.; Fierer, J. D-Galactosamine hepatotoxicity is associated with endotoxin sensitivity and mediated by lymphoreticular cells in mice. Gastroenterology 1985, 88, 115–121. [Google Scholar] [CrossRef]
- Keppler, D.; Lesch, R.; Reutter, W.; Decker, K. Experimental hepatitis induced by D-galactosamine. Exp. Mol. Pathol. 1968, 9, 279–290. [Google Scholar] [CrossRef]
- Lesch, R.; Reutter, W.; Keppler, D.; Decker, K. Liver restitution after acute galactosamine hepatitis: Autoradiographic and biochemical studies in rats. Exp. Mol. Pathol. 1969, 12, 58–69. [Google Scholar] [CrossRef]
- Eipel, C.; Kidess, E.; Abshagen, K.; LeMinh, K.; Menger, M.D.; Burkhardt, H.; Vollmar, B. Antileukoproteinase protects against hepatic inflammation, but not apoptosis in the response of D-galactosamine-sensitized mice to lipopolysaccharide. Br. J. Pharmacol. 2007, 151, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Mayer, A.M.; Spitzer, J.A. Modulation of superoxide anion generation by manoalide, arachidonic acid and staurosporine in liver infiltrated neutrophils in a rat model of endotoxemia. J. Pharmacol. Exp. Ther. 1993, 267, 400–409. [Google Scholar]
- Yang, F.; Li, X.; Wang, L.K.; Wang, L.W.; Han, X.Q.; Zhang, H.; Gong, Z.J. Inhibitions of NF-κB and TNF-α result in differential effects in rats with acute on chronic liver failure induced by d-Gal and LPS. Inflammation 2014, 37, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Wadleigh, D.J.; Reddy, S.; Kopp, T.E.; Ghosh, S.; Herschman, H.R. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J. Biol. Chem. 2000, 275, 6259–6266. [Google Scholar] [CrossRef] [Green Version]
- Cheng, B.C.; Ma, X.Q.; Kwan, H.Y.; Tse, K.W.; Cao, H.H.; Su, T.; Shu, X.; Wu, Z.Z.; Yu, Z.L. A herbal formula consisting of Rosae Multiflorae Fructus and Lonicerae Japonicae Flos inhibits inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2014, 153, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, R. AP-1: One switch for many signals. Exp. Cell Res. 1999, 253, 180–185. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Otterbein, L.E.; Morse, D.; Choi, A.M. Heme oxygenase/carbon monoxide signaling pathways: Regulation and functional significance. Mol. Cell Biochem. 2002, 234, 249–263. [Google Scholar]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Yim, N.H.; Ma, J.Y. Hoveniae Semen Seu Fructus Ethanol Extract Exhibits Anti-Inflammatory Activity via MAPK, AP-1, and STAT Signaling Pathways in LPS-Stimulated RAW 264.7 and Mouse Peritoneal Macrophages. Mediators Inflamm. 2019, 2019, 9184769. [Google Scholar] [CrossRef] [Green Version]
- Piao, X.L.; Jang, M.H.; Cui, J.; Piao, X. Lignans from the fruits of Forsythia suspensa. Bioorg. Med. Chem. Lett. 2008, 18, 1980–1984. [Google Scholar] [CrossRef]
- Bae, W.Y.; Kim, H.Y.; Yu, H.S.; Chang, K.H.; Hong, Y.H.; Lee, N.K.; Paik, H.D. Antimicrobial effects of three herbs (Brassica juncea, Forsythia suspensa, and Inula britannica) on membrane permeability and apoptosis in Salmonella. J. Appl. Microbiol. 2021, 130, 394–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, F.; Chen, T.; Li, Z.; Shen, Q.W. Antidiabetic and antihyperlipidemic activities of Forsythia suspensa (Thunb.) Vahl (fruit) in streptozotocin-induced diabetes mice. J. Ethnopharmacol. 2016, 192, 256–263. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, S.W.; Cho, S.H.; Rhee, S.J. Effect of Forsythia Viridissima Extracts on Antioxidative System and Lipid Peroxidation of Liver in Rats Fed High-Cholesterol Diet. Korean J. Nutr. 2003, 36, 990–996. [Google Scholar]
- Wang, L.; Piao, X.L.; Kim, S.W.; Piao, X.S.; Shen, Y.B.; Lee, H.S. Effects of Forsythia suspensa extract on growth performance, nutrient digestibility, and antioxidant activities in broiler chickens under high ambient temperature. Poult. Sci. 2008, 87, 1287–1294. [Google Scholar] [CrossRef]
- Guo, H.; Liu, A.H.; Li, L.; Guo, D.A. Simultaneous determination of 12 major constituents in Forsythia suspensa by high performance liquid chromatography--DAD method. J. Pharm. Biomed. Anal. 2007, 43, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Cho, S.H.; Sin, E.N.; Choi, K.H.; Choi, Y.S. Effects of alcohol consumption and fat content in diet on chemical composition and morphology of liver in rat. Korean J. Nutr. 1988, 21, 154–163. [Google Scholar]
- Yi, Y.S.; Cho, J.Y.; Kim, D. Cerbera manghas methanol extract exerts anti-inflammatory activity by targeting c-Jun N-terminal kinase in the AP-1 pathway. J. Ethnopharmacol. 2016, 193, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Choi, R.J.; Khan, S.; Lee, D.S.; Kim, Y.C.; Nam, Y.J.; Lee, D.U.; Kim, Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-kappaB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int. Immunopharmacol. 2012, 14, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, C.W.; Zhou, G.Y.; Chen, W.L.; Zhuge, L.; Jin, L.X.; Zheng, Y.; Lin, W.; Pan, Z.Z. Protective effect of forsythiaside A on lipopolysaccharide/d-galactosamine-induced liver injury. Int. Immunopharmacol. 2015, 26, 80–85. [Google Scholar] [CrossRef]
- Gong, L.; Zhou, H.; Wang, C.; He, L.; Guo, C.; Peng, C.; Li, Y. Hepatoprotective effect of forsythiaside a against acetaminophen-induced liver injury in zebrafish: Coupling network pharmacology with biochemical pharmacology. J. Ethnopharmacol. 2021, 271, 113890. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Lin, C.; Ren, J.; Zhang, S. Forsythiaside A Exhibits Anti-inflammatory Effects in LPS-Stimulated BV2 Microglia Cells Through Activation of Nrf2/HO-1 Signaling Pathway. Neurochem. Res. 2016, 41, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, J.K.; Choi, J.H.; Jung, J.Y.; Oh, W.Y.; Kim, D.C.; Lee, H.S.; Kim, Y.S.; Kang, S.S.; Lee, S.H.; et al. Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice. J. Pharmacol. Sci. 2010, 112, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Wang, C.; Dai, X.; Zhou, M.; Gong, L.; Yu, L.; Peng, C.; Li, Y. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-kappaB signaling pathway. J. Ethnopharmacol. 2020, 248, 112361. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wu, J.; Yu, L.; Peng, Z. Evaluation of the Pharmacokinetics and Hepatoprotective Effects of Phillygenin in Mouse. Biomed. Res. Int. 2018, 2018, 7964318. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Target Gene | Primer Sequence |
|---|---|
| TNF-α | F: 5′-TTCTGTCTACTGAACTTCGGGGTGATCGGTCC-3′ |
| R: 5′-GTATGAGATAGCAAATCGGCTGACGGTGTGGG-3′ | |
| IL-6 | F: 5′-TCCAGTTGCCTTCTTGGGAC-3′ |
| R: 5′-GTGTAATTAAGCCTCCGACTTG-3′ | |
| IL-1β | F: 5′-ATGGCAACTGTTCCTGAACTCAACT-3′ |
| R: 5′-CAGGACAGGTATAGATTCTTTCCTTT-3′ | |
| β-actin | F: 5′-AGAGGGAAATCGTGCGTGAC-3′ |
| R: 5′-CAATAGTGATGACCTGGCCGT-3′ |
| Antibody | Corporation | Product No. | RRID | Dilution Rate |
|---|---|---|---|---|
| iNOS | Cell Signaling | #13120 | AB_2687529 | 1:1000 |
| COX-2 | Cell Signaling | #4842 | AB_2085144 | 1:1000 |
| HO-1 | Cell Signaling | #82206 | AB_2799989 | 1:1000 |
| Nrf-2 | Cell Signaling | #12721 | AB_2715528 | 1:1000 |
| P-NF-κB p65 | Cell Signaling | #3033 | AB_331284 | 1:1000 |
| P-IκBα | Cell Signaling | #2859 | AB_561111 | 1:1000 |
| IκBα | Cell Signaling | #4814 | AB_390781 | 1:1000 |
| P-ERK | Cell Signaling | #4377 | AB_331775 | 1:1000 |
| ERK | Cell Signaling | #9102 | AB_330744 | 1:1000 |
| P-p38 | Cell Signaling | #9211 | AB_331641 | 1:1000 |
| p38 | Cell Signaling | #9212 | AB_330713 | 1:1000 |
| P-JNK | Cell Signaling | #9251 | AB_331659 | 1:1000 |
| JNK | Cell Signaling | #9252 | AB_2250373 | 1:1000 |
| β-actin | Cell Signaling | #4970 | AB_2223172 | 1:1000 |
| TBP | Cell Signaling | #8515 | AB_10949159 | 1:1000 |
| 2nd anti-mouse | Cell Signaling | #7076 | AB_330924 | 1:5000 |
| 2nd anti-rabbit | Cell Signaling | #7074 | AB_2099233 | 1:5000 |
| HPLC Conditions | |||
|---|---|---|---|
| Detector | 280 nm | ||
| Column | X bridge C18 Column (250 mm × 4.6 mm, 5 μm) | ||
| Column temperature | 40°C | ||
| Injection volume | 10 μL | ||
| Flow rate | 1.0 mL/min | ||
| Mobile phase | Time (min) | A | B |
| A: 0.3% acetic acid in water B: MeOH | 0.0 | 95 | 5 |
| 8.0 | 70 | 30 | |
| 24.0 | 43 | 57 | |
| 39.0 | 40 | 60 | |
| 50.0 | 30 | 70 | |
| 60.0 | 0 | 100 | |
| Compound | Range (μg/mL) | Regression Equation | r2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| 1 | 200.0~500.0 | y = 0.2516x − 3.8826 | 0.9958 | 0.0527 | 0.1598 |
| 2 | 20.0~200.0 | y = 0.1132x + 0.1922 | 0.9990 | 0.0879 | 0.2664 |
| 3 | 2.5~25.0 | y = 0.1927x + 0.0909 | 0.9994 | 0.0517 | 0.1565 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.H.; Hwang, Y.-H.; Kim, T.I.; Oh, Y.-C.; Ma, J.Y. Forsythia Fruit Prevents Fulminant Hepatitis in Mice and Ameliorates Inflammation in Murine Macrophages. Nutrients 2021, 13, 2901. https://doi.org/10.3390/nu13082901
Jeong YH, Hwang Y-H, Kim TI, Oh Y-C, Ma JY. Forsythia Fruit Prevents Fulminant Hepatitis in Mice and Ameliorates Inflammation in Murine Macrophages. Nutrients. 2021; 13(8):2901. https://doi.org/10.3390/nu13082901
Chicago/Turabian StyleJeong, Yun Hee, Youn-Hwan Hwang, Tae In Kim, You-Chang Oh, and Jin Yeul Ma. 2021. "Forsythia Fruit Prevents Fulminant Hepatitis in Mice and Ameliorates Inflammation in Murine Macrophages" Nutrients 13, no. 8: 2901. https://doi.org/10.3390/nu13082901
APA StyleJeong, Y. H., Hwang, Y. -H., Kim, T. I., Oh, Y. -C., & Ma, J. Y. (2021). Forsythia Fruit Prevents Fulminant Hepatitis in Mice and Ameliorates Inflammation in Murine Macrophages. Nutrients, 13(8), 2901. https://doi.org/10.3390/nu13082901