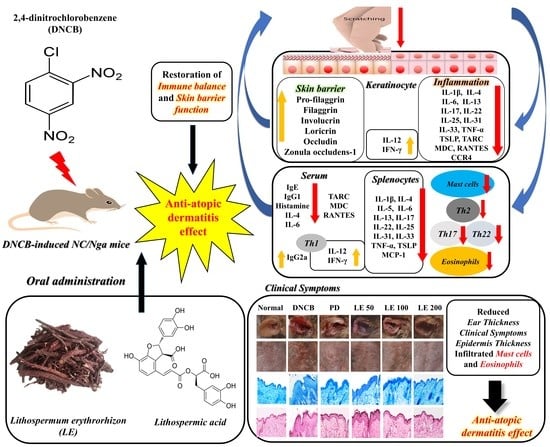
Lithospermum erythrorhizon Alleviates Atopic Dermatitis-like Skin Lesions by Restoring Immune Balance and Skin Barrier Function in 2.4-Dinitrochlorobenzene-Induced NC/Nga Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the LE Extract
2.2. High-Performance Liquid Chromatography (HPLC) Analysis of LE
2.3. Animals
2.4. Induction of AD-like Skin Lesions and LE Treatment
2.5. Dermatitis Score and Ear Thickness
2.6. Scratching Behavior
2.7. Histological Analysis
2.8. Serum Immunoglobulin and Histamine Assay
2.9. Serum Cytokines and Chemokines Assay
2.10. Isolation of Splenocytes and Analysis of Cytokines and Chemokines
2.11. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.12. Western Blotting
2.13. Statistical Analysis
3. Results
3.1. Identification and Qualification of Lithospermic Acid in the LE Extracts
3.2. LE Attenuates DNCB-Induced AD-like Symptoms and Scratching Behavior
3.3. LE Reduces Epidermal Thickening as Well as Eosinophil and Mast Cell Infiltration
3.4. LE Decreases IL-4, IgE, and Histamine Serum Levels
3.5. LE Restores Th1/Th2 Immune Balance by Regulating Serum IgG1 and IgG2a Levels in NC/Nga Mice
3.6. LE Decreases the Levels of AD-Related Cytokines and Chemokines in NC/Nga Mouse Serum
3.7. LE Regulates the Balance of Cytokines and Chemokines Secretion in Splenocytes
3.8. LE Inhibits the Gene Expression of Cytokines, Chemokines, and CCR4 Involved in Th2 Activation in the Dorsal Skin
3.9. LE Restores the Balance of AD-Related Cytokine and Chemokine Gene Expression in the Dorsal Skin of the NC/Nga Mouse
3.10. LE Restores Defects in Skin Barrier Function Caused by AD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flohr, C.; Mann, J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014, 69, 3–16. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A Novel Atopic Dermatitis Model Induced by Topical Application with Dermatophagoides Farinae Extract in NC/Nga Mice. Allergol. Int. 2007, 56, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin In-flammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function. Nutrients 2020, 12, 249. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Va-siadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, E.B.; Sivaprasad, U. Th2 Cytokines and Atopic Dermatitis. J. Clin. Cell. Immunol. 2011, 2, 3. [Google Scholar] [CrossRef]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Nomura, T.; Common, J.; Kabashima, K. Insights into atopic dermatitis gained from genetically defined mouse models. J. Allergy Clin. Immunol. 2019, 143, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, J.S.; Cho, D.H.; Park, H.J. Molecular Mechanisms of Cutaneous Inflammatory Disorder: Atopic Der-matitis. Int. J. Mol. Sci. 2016, 17, 1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Choi, M.J.; Bak, D.H.; Lee, B.C.; Ko, E.J.; Ahn, G.R.; Ahn, S.W.; Kim, M.J.; Na, J.; Kim, B.J. Topical admin-istration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 2018, 8, 11895. [Google Scholar] [CrossRef] [Green Version]
- Danso, M.O.; van Drongelen, V.; Mulder, A.; van Esch, J.; Scott, H.; van Smeden, J.; El Ghalbzouri, A.; Bouwstra, J.A. TNF-α and Th2 Cytokines Induce Atopic Dermatitis–Like Features on Epidermal Differentiation Proteins and Stratum Corneum Lipids in Human Skin Equivalents. J. Investig. Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef] [Green Version]
- Howell, M.D.; Fairchild, H.R.; Kim, B.E.; Bin, L.; Boguniewicz, M.; Redzic, J.S.; Hansen, K.C.; Leung, D.Y. Th2 Cytokines Act on S100/A11 to Downregulate Keratinocyte Differentiation. J. Investig. Dermatol. 2008, 128, 2248–2258. [Google Scholar] [CrossRef] [Green Version]
- Noben-Trauth, N.; Hu-Li, J.; Paul, W.E. Conventional, naive CD4+ T cells provide an initial source of IL-4 during Th2 differentiation. J. Immunol. 2000, 165, 3620–3625. [Google Scholar] [CrossRef] [Green Version]
- Paludan, S.R. Interleukin-4 and interferon-gamma: The quintessence of a mutual antagonistic relationship. Scand. J. Immunol. 1998, 48, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.K.; Hummelshoj, L. Triggers of IgE class switching and allergy development. Ann. Med. 2007, 39, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Hwang, M.H.; Cho, Y.-R.; Hong, S.S.; Kang, J.-S.; Kim, W.H.; Yang, S.H.; Seo, D.-W.; Oh, J.S.; Ahn, E.-K. Combretum quadrangulare Extract Attenuates Atopic Dermatitis-Like Skin Lesions through Modulation of MAPK Signaling in BALB/c Mice. Molecules 2020, 25, 2003. [Google Scholar] [CrossRef]
- Badloe, F.M.S.; De Vriese, S.; Coolens, K.; Schmidt-Weber, C.B.; Ring, J.; Gutermuth, J.; Kortekaas Krohn, I. IgE auto-antibodies and autoreactive T cells and their role in children and adults with atopic dermatitis. Clin. Transl. Allergy 2020, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemény, L.; Alenius, H.; Dieu-Nosjean, M.-C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef]
- Furue, M. T helper type 2 signatures in atopic dermatitis. J. Cutan. Immunol. Allergy 2018, 1, 93–99. [Google Scholar] [CrossRef]
- Nygaard, U.; Hvid, M.; Johansen, C.; Buchner, M.; Fölster-Holst, R.; Deleuran, M.; Vestergaard, C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1930–1938. [Google Scholar] [CrossRef]
- Han, H.; Roan, F.; Ziegler, S.F. The atopic march: Current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 2017, 278, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Čepelak, I.; Dodig, S.; Pavić, I. Filaggrin and atopic march. Biochem. Med. 2019, 29, 214–227. [Google Scholar] [CrossRef]
- Honzke, S.; Wallmeyer, L.; Ostrowski, A.; Radbruch, M.; Mundhenk, L.; Schafer-Korting, M.; Hedtrich, S. Influence of Th2 Cytokines on the Cornified Envelope, Tight Junction Proteins, and ss-Defensins in Filaggrin-Deficient Skin Equivalents. J. Investig. Dermatol. 2016, 136, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minai-Fleminger, Y.; Levi-Schaffer, F. Mast cells and eosinophils: The two key effector cells in allergic inflammation. Inflamm. Res. 2009, 58, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Okada, M.; Hara, M.; Furukawa, Y.; Matsumori, A. Mast Cell Tryptase in Mast Cell Granules Enhances MCP-1 and Interleukin-8 Production in Human Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1858–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Tseng, S.; Horner, R.M.; Tam-Amersdorfer, C.; Loda, M.; Rollins, B.J. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 2000, 404, 407–411. [Google Scholar] [CrossRef]
- Imai, T.; Chantry, D.; Raport, C.J.; Wood, C.L.; Nishimura, M.; Godiska, R.; Yoshie, O.; Gray, P.W. Macrophage-derived Chemokine Is a Functional Ligand for the CC Chemokine Receptor 4. J. Biol. Chem. 1998, 273, 1764–1768. [Google Scholar] [CrossRef] [Green Version]
- Aust, G.; Simchen, C.; Heider, U.; Hmeidan, F.A.; Blumenauer, V.; Spanel-Borowski, K. Eosinophils in the human corpus luteum: The role of RANTES and eotaxin in eosinophil attraction into periovulatory structures. Mol. Hum. Reprod. 2000, 6, 1085–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.; Seong, G.; Kim, Y.; Choung, S. Effects of Deacetylasperulosidic Acid on Atopic Dermatitis through Modulating Immune Balance and Skin Barrier Function in HaCaT, HMC-1, and EOL-1 Cells. Molecules 2021, 26, 3298. [Google Scholar] [CrossRef]
- Zaniboni, M.C.; Samorano, L.P.; Orfali, R.L.; Aoki, V. Skin barrier in atopic dermatitis: Beyond filaggrin. An. Bras. Dermatol. 2016, 91, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Novak, N. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 2016, 42, 1–8. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y. Epidermal Barrier in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2012, 4, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Wu, Q.; Jang, Y.P.; Choung, S.Y. Pinus densiflora bark extract ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by regulating Th1/Th2 balance and skin barrier function. Phytother. Res. 2018, 32, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cork, M.J.; Danby, S.; Vasilopoulos, Y.; Hadgraft, J.; Lane, M.E.; Moustafa, M.; Guy, R.; MacGowan, A.L.; Tazi-Ahnini, R.; Ward, S.J. Epidermal Barrier Dysfunction in Atopic Dermatitis. J. Investig. Dermatol. 2009, 129, 1892–1908. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Steinert, P.M.; Kartasova, T.; Marekov, L.N. Biochemical Evidence That Small Proline-rich Proteins and Trichohyalin Function in Epithelia by Modulation of the Biomechanical Properties of Their Cornified Cell Envelopes. J. Biol. Chem. 1998, 273, 11758–11769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segre, J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006, 116, 1150–1158. [Google Scholar] [CrossRef]
- Candi, E.; Melino, G.; Mei, G.; Tarcsa, E.; Chung, S.-I.; Marekov, L.N.; Steinert, P.M. Biochemical, Structural, and Transglutaminase Substrate Properties of Human Loricrin, the Major Epidermal Cornified Cell Envelope Protein. J. Biol. Chem. 1995, 270, 26382–26390. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niessen, C.M. Tight junctions/adherens junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2525–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J. The Tight Junction Protein ZO-1 Establishes a Link between the Transmembrane Protein Occludin and the Actin Cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [Green Version]
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Skin Barrier and Immune Dysregulation in Atopic Dermatitis: An Evolving Story with Important Clinical Implications. J. Allergy Clin. Immunol. Pract. 2014, 2, 371–379. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [Green Version]
- Gittler, J.K.; Shemer, A.; Suarez-Farinas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Scherer, A.; Wanke, C.; Bräutigam, M.; Bongiovanni, S.; Letzkus, M.; Staedtler, F.; Kehren, J.; Zuehlsdorf, M.; Schwarz, T.; et al. Gene expression is differently affected by pimecrolimus and betamethasone in lesional skin of atopic dermatitis. Allergy 2011, 67, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Carr, W.W. Topical Calcineurin Inhibitors for Atopic Dermatitis: Review and Treatment Recommendations. Pediatr. Drugs 2013, 15, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Takeuchi, H.; Sakuma, S.; Sengoku, T.; Takakura, S. Characterization of a 2,4-Dinitrochlorobenzene-Induced Chronic Dermatitis Model in Rats. Skin Pharmacol. Physiol. 2009, 22, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Ichino, T.; Onishi, N.; Shimomura, K.; Yazaki, K. Highly efficient method of Lithospermum erythrorhizon transformation using domestic Rhizobium rhizogenes strain A13. Plant Biotechnol. 2020, 37, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Yazaki, K. Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnol. 2017, 34, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Suto, H.; Matsuda, H.; Mitsuishi, K.; Hira, K.; Uchida, T.; Unno, T.; Ogawa, H.; Ra, C. NC/Nga Mice: A Mouse Model for Atopic Dermatitis. Int. Arch. Allergy Immunol. 1999, 120, 70–75. [Google Scholar] [CrossRef]
- Takano, N.; Arai, I.; Kurachi, M. Analysis of the spontaneous scratching behavior by NC/Nga mice: A possible approach to evaluate antipruritics for subjects with atopic dermatitis. Eur. J. Pharmacol. 2003, 471, 223–228. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Maurelli, M.; Peris, K.; Girolomoni, G. Targeting IL-4 for the Treatment of Atopic Dermatitis. ImmunoTargets Ther. 2020, 9, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Voisin, T.; Chiu, I.M. Molecular link between itch and atopic dermatitis. Proc. Natl. Acad. Sci. USA 2018, 115, 12851–12853. [Google Scholar] [CrossRef] [Green Version]
- Siegfried, E.C.; Hebert, A.A. Diagnosis of Atopic Dermatitis: Mimics, Overlaps, and Complications. J. Clin. Med. 2015, 4, 884–917. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.; Kim, M.; Ha, I.; Yang, W. Derma-Hc, a New Developed Herbal Formula, Ameliorates Cutaneous Lichenification in Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 2359. [Google Scholar] [CrossRef]
- Yarbrough, K.B.; Neuhaus, K.J.; Simpson, E.L. The effects of treatment on itch in atopic dermatitis. Dermatol. Ther. 2013, 26, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.; Trujillo-Paez, J.; Umehara, Y.; Yue, H.; Peng, G.; Kiatsurayanon, C.; Chieosilapatham, P.; Song, P.; Okumura, K.; Ogawa, H.; et al. Role of Antimicrobial Peptides in Skin Barrier Repair in Individuals with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 7607. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.S.; McPhee, R.; Arruda, L.K.; Howell, M.D. Targeting the T Helper 2 Inflammatory Axis in Atopic Dermatitis. Int. Arch. Allergy Immunol. 2016, 171, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Paez, J.V.T.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.; Henze, S.; Wörenkämper, E.; Basner-Tschakarjan, E.; Sokolowska-Wojdylo, M.; Steitz, J.; Bieber, T.; Tüting, T. Role of the Chemokine Receptor CCR4 and its Ligand Thymus- and Activation-Regulated Chemokine/CCL17 for Lymphocyte Recruitment in Cutaneous Lupus Erythematosus. J. Investig. Dermatol. 2005, 124, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, B.F.; Patsinakidis, N.; Raap, U. Role of the Pruritic Cytokine IL-31 in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 1383. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Nograles, K.E.; Krueger, J.G. Contrasting pathogenesis of atopic dermatitis and psoriasis—Part II: Immune cell subsets and therapeutic concepts. J. Allergy Clin. Immunol. 2011, 127, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jak-Stat 2013, 2, e24137. [Google Scholar] [CrossRef] [Green Version]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Ruckemann-Dziurdzińska, K.; Roszkiewicz, J.; Nowicki, R.J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Postȩpy Dermatol. Alergol. 2014, 2, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; He, C.; Nair, L.; Yeung, J.; Egwuagu, C.E. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015, 75, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Nakayamada, S.; Kubo, S.; Nakano, K.; Iwata, S.; Miyagawa, I.; Ma, X.; Trimova, G.; Sakata, K.; Tanaka, Y. Th22 Cells Promote Osteoclast Differentiation via Production of IL-22 in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 2901. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Sun, L.; Jiang, T.; Zhang, D.; He, D.; Nie, H. TNFalpha promotes Th17 cell differentiation through IL-6 and IL-1beta produced by monocytes in rheumatoid arthritis. J. Immunol. Res. 2014, 2014, 385352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biedermann, T.; Skabytska, Y.; Kaesler, S.; Volz, T. Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front. Immunol. 2015, 6, 353. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Son, K.-H.; Park, H.-Y.; Kim, M.J.; Shin, D.-H.; Lee, J.-S. Arazyme inhibits cytokine expression and upregulates skin barrier protein expression. Mol. Med. Rep. 2013, 8, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Iwamoto, K.; Moriwaki, M.; Miyake, R.; Hide, M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. 2019, 68, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Ungar, B.; Malik, K.; Dickstein, D.; Suprun, M.; Estrada, Y.D.; Xu, H.; Peng, X.; Oliva, M.; Todd, D.; et al. Molecular signatures order the potency of topically applied anti-inflammatory drugs in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2017, 140, 1032–1042. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Kubo, A.; Fujita, H.; Yokouchi, M.; Ishii, K.; Kawasaki, H.; Nomura, T.; Shimizu, H.; Kouyama, K.; Ebihara, T.; et al. Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Choi, Y.; Kim, S.-E.; Noh, E.B.; Kim, S.-C. Differential effects of topical corticosteroid and calcineurin inhibitor on the epidermal tight junction. Exp. Dermatol. 2012, 22, 59–61. [Google Scholar] [CrossRef] [PubMed]










| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| IL-1β (m) | TGT GTT TTC CTC CTT GCC TCT GAT | TGC TGC CTA ATG TCC CCT TGA AT |
| IL-4 (m) | ACG GAG ATG GAT GTG CCA AAC | AGC ACC TTG GAA GCC CTA CAG A |
| IL-5 (m) | TCA GCT GTG TCT GGG CCA CT | TT ATG AGT AGG GAC AGG AAG CCT CA |
| IL-6 (m) | CCA CTT CAC AAG TCG GAG GCT TA | GCA AGT GCA TCA TCG TTG TTC ATA C |
| IL-12 (m) | TGA ACT GGC GTT GGA AGC | GCG GGT CTG GTT TGA TGA |
| IL-13 (m) | CAA TTG CAA TGC CAT CTA CAG GAC | CGA AAC AGT TGC TTT GTG TAG CTG A |
| IL-17 (m) | AAG GCA GCA GCG ATC ATC C | GGA ACG GTT GAG GTA GTC TGA G |
| IL-22 (m) | CAG CTC CTG TCA CAT CAG CGG T | AGG TCC AGT TCC CCA ATC GCC T |
| IL-25 (m) | CTC AAC AGC AGG GCC ACT C | GTC TGT AGG CTG ACG CAG TGT G |
| IL-31 (m) | ATA CAG CTG CCG TGT TTC AG | AGC CAT CTT ATC ACC CAA GAA |
| IL-33 (m) | GAT GAG ATG TCT CGG CTG CTT G | AGC CGT TAC GGA TAT GGT GGT C |
| IFN-γ (m) | CGG CAC AGT CAT TGA AAG CCT A | GGC ACC ACT AGT TGG TTG TCT TTG |
| TNF-α (m) | TAC TGA ACT TCG GGG TGA TTG GTC | CAG CCT TGT CCC TTG AAG AGA ACC |
| TSLP (m) | TGC AAG TAC TAG TAC GGA TGG GGC | GGA CTT CTT GTG CCA TTT CCT GAG |
| TARC (m) | TGA GGT CAC TTC AGA TGC TGC | ACC AAT CTG ATG GCC TTC TTC |
| MDC (m) | CAG GCA GGT CTG GGT GAA | TAA AGG TGG CGT CGT TGG |
| RANTES (m) | GGA GTA TTT CTA CAC CAG CAG CAA | GGC TAG GAC TAG AGC AAG CAA TGA C |
| CCR4 (m) | TCT ACA GCG GCA TCT TCT TCA T | CAG TAC GTG TGG TGG TGC TCT G |
| Pro-filaggrin (m) | GAA TCC ATA TTT ACA GCA AAG CAC CTT G | GGT ATG TCC AAT GTG ATT GCA CGA TTG |
| GAPDH (m) | ACT TTG TCA AGC TCA TTT CC | TGC AGC GAA CTT TAT TGA TG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-S.; Lee, S.-J.; Choung, S.-Y. Lithospermum erythrorhizon Alleviates Atopic Dermatitis-like Skin Lesions by Restoring Immune Balance and Skin Barrier Function in 2.4-Dinitrochlorobenzene-Induced NC/Nga Mice. Nutrients 2021, 13, 3209. https://doi.org/10.3390/nu13093209
Oh J-S, Lee S-J, Choung S-Y. Lithospermum erythrorhizon Alleviates Atopic Dermatitis-like Skin Lesions by Restoring Immune Balance and Skin Barrier Function in 2.4-Dinitrochlorobenzene-Induced NC/Nga Mice. Nutrients. 2021; 13(9):3209. https://doi.org/10.3390/nu13093209
Chicago/Turabian StyleOh, Jin-Su, Sang-Jun Lee, and Se-Young Choung. 2021. "Lithospermum erythrorhizon Alleviates Atopic Dermatitis-like Skin Lesions by Restoring Immune Balance and Skin Barrier Function in 2.4-Dinitrochlorobenzene-Induced NC/Nga Mice" Nutrients 13, no. 9: 3209. https://doi.org/10.3390/nu13093209
APA StyleOh, J. -S., Lee, S. -J., & Choung, S. -Y. (2021). Lithospermum erythrorhizon Alleviates Atopic Dermatitis-like Skin Lesions by Restoring Immune Balance and Skin Barrier Function in 2.4-Dinitrochlorobenzene-Induced NC/Nga Mice. Nutrients, 13(9), 3209. https://doi.org/10.3390/nu13093209