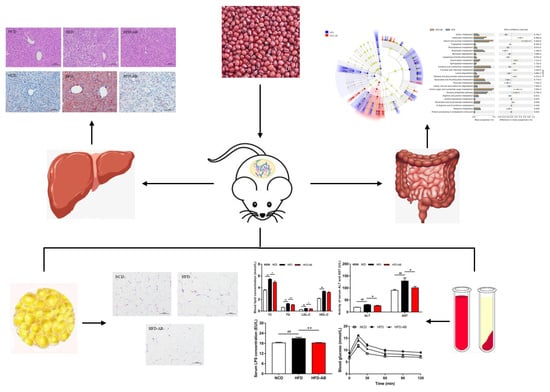
Adzuki Bean Alleviates Obesity and Insulin Resistance Induced by a High-Fat Diet and Modulates Gut Microbiota in Mice
Abstract
:1. Introduction
2. Methods
2.1. Material Preparation
2.2. Animals and Diets
2.3. Biochemical Analysis
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Histological Analysis
2.6. Gut Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Adzuki Bean Supplementation Alleviated Obesity Induced by HFD
3.2. Effect of Adzuki Bean Supplementation on Serum Parameters
3.3. Adzuki Bean Supplementation Limited Hepatic Steatosis and Lipid Accumulation
3.4. Adzuki Bean Supplementation Improved Insulin Resistance Induced by HFD
3.5. Adzuki Bean Supplementation Regulated Gut Microbiota Dysbiosis
3.6. Gut Microbial Metabolic Functions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care 2016, 43, 121–135. [Google Scholar] [CrossRef]
- Moreira, R.E., Jr.; de Carvalho, L.M.; Reis, D.C.D.; Cassali, G.D.; Faria, A.M.C.; Maioli, T.U.; Brunialti-Godard, A.L. Diet-induced obesity leads to alterations in behavior and gut microbiota composition in mice. J. Nutr. Biochem. 2021, 92, 108622. [Google Scholar] [CrossRef]
- Liu, J.; Cao, J.; Li, Y.; Guo, F. Beneficial Flavonoid in Foods and Anti-obesity Effect. Food Rev. Int. 2021, 1–41. [Google Scholar] [CrossRef]
- Moreira, R.E., Jr.; de Carvalho, L.M.; Pedersen, A.S.B.; Damasceno, S.; Maioli, T.U.; de Faria, A.M.C.; Godard, A.L.B. Interaction between high-fat diet and ethanol intake leads to changes on the fecal microbiome. J. Nutr. Biochem. 2019, 72, 108215. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-H.; Ohashi, S.; Sasaki, K.; Nagata, R.; Pelpolage, S.; Fukuma, N.; Reed, J.D.; Shimada, K.-I.; Kadoya, N.; Fukushima, M. Dietary adzuki bean paste dose-dependently reduces visceral fat accumulation in rats fed a normal diet. Food Res. Int. 2020, 130, 108890. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Khan, J.; Xue, Y.; Shen, Q. Consumption of mung bean (Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet. J. Funct. Foods 2020, 64, 103687. [Google Scholar] [CrossRef]
- Kitano-Okada, T.; Ito, A.; Koide, A.; Nakamura, Y.; Han, K.-H.; Shimada, K.; Sasaki, K.; Ohba, K.; Sibayama, S.; Fukushima, M. Anti-obesity role of adzuki bean extract containing polyphenols: In vivo and in vitro effects. J. Sci. Food Agric. 2012, 92, 2644–2651. [Google Scholar] [CrossRef]
- Li, L.; Yang, T.; Liu, R.; Redden, B.; Maalouf, F.; Zong, X. Food legume production in China. Crop. J. 2017, 5, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Rui, L. Anti-Obesity Effects of Flavonoids and Saponins from Adzuki Bean. Ph.D. Thesis, Hong Kong Baptist University, Hong Kong, China, 2014. [Google Scholar]
- Wu, G.; Bai, Z.; Wan, Y.; Shi, H.; Huang, X.; Nie, S. Antidiabetic effects of polysaccharide from azuki bean (Vigna angularis) in type 2 diabetic rats via insulin/PI3K/AKT signaling pathway. Food Hydrocoll. 2020, 101, 105456. [Google Scholar] [CrossRef]
- Ashraf, J.; Awais, M.; Liu, L.; Khan, M.I.; Tong, L.-T.; Ma, Y.; Wang, L.; Zhou, X.; Zhou, S. Effect of thermal processing on cholesterol synthesis, solubilisation into micelles and antioxidant activities using peptides of Vigna angularis and Vicia faba. LWT 2020, 129, 109504. [Google Scholar] [CrossRef]
- Kim, S.; Hong, J.; Jeon, R.; Kim, H.-S. Adzuki bean ameliorates hepatic lipogenesis and proinflammatory mediator expression in mice fed a high-cholesterol and high-fat diet to induce nonalcoholic fatty liver disease. Nutr. Res. 2016, 36, 90–100. [Google Scholar] [CrossRef]
- Durak, A.; Baraniak, B.; Jakubczyk, A.; Świeca, M. Biologically active peptides obtained by enzymatic hydrolysis of Adzuki bean seeds. Food Chem. 2013, 141, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hou, D.; Yousaf, L.; Xue, Y.; Shen, Q. Comparison of the effects of raw and cooked adzuki bean on glucose/lipid metabolism and liver function in diabetic mice. Cereal Chem. J. 2021, 98, 1081–1090. [Google Scholar] [CrossRef]
- Wang, J. Effect and Mechanism of Ginger on Energy Metabolism and Gut Microflora in Obese mice. Ph.D. Thesis, China Agricultural University, Beijing, China, 2019. [Google Scholar]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, J.; Zhang, L.; Liu, P.; Zheng, P.; Gao, S.; Song, C.; Yu, Y.; Gong, Z.; Wan, X. Gut microbiota-mediated improvement of metabolic disorders by Qingzhuan tea in high fat diet-fed mice. J. Funct. Foods 2021, 78, 104366. [Google Scholar] [CrossRef]
- Lee, P.; Teng, C.; Hsieh, K.; Chiou, Y.; Wu, J.; Lu, T.; Pan, M. Adzuki Bean Water Extract Attenuates Obesity by Modulating M2/M1 Macrophage Polarization and Gut Microbiota Composition. Mol. Nutr. Food Res. 2019, 63, e1900626. [Google Scholar] [CrossRef]
- Ellenbroek, J.H.; van Dijck, L.; Tons, H.A.; Rabelink, T.J.; Carlotti, F.; Ballieux, B.; de Koning, E.J.P. Long-term ketogenic diet causes glucose intolerance and reduced beta- and alpha-cell mass but no weight loss in mice. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E552–E558. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.-J.; Liu, D.; Wan, Y.-J.; Huang, X.-J.; Nie, S.-P. Comparison of hypoglycemic effects of polysaccharides from four legume species. Food Hydrocoll. 2019, 90, 299–304. [Google Scholar] [CrossRef]
- Yao, Y.; Ren, G. Suppressive effect of extruded adzuki beans (Vigna angularis) on hyperglycemia after sucrose loading in rats. Ind. Crop. Prod. 2014, 52, 228–232. [Google Scholar] [CrossRef]
- Guo, B.; Yang, B.; Pang, X.; Chen, T.; Chen, F.; Cheng, K.-W. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019, 10, 5644–5655. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, Y.; Chen, X.; Zhou, H.; Yang, Y.; Zhang, X.; Huang, Y.; Zhang, N.; Lui, E.M.; Xiao, M. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res. Int. 2021, 144, 110360. [Google Scholar] [CrossRef]
- Fang, D.; Wang, D.; Ma, G.; Ji, Y.; Zheng, H.; Chen, H.; Zhao, M.; Hu, Q.; Zhao, L. Auricularia polytricha noodles prevent hyperlipemia and modulate gut microbiota in high-fat diet fed mice. Food Sci. Hum. Wellness 2021, 10, 431–441. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonné, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2018, 68, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Xue, Y.; Shen, Q. Whole mung bean (Vigna radiata L.) supplementation prevents high-fat diet-induced obesity and disorders in a lipid profile and modulates gut microbiota in mice. Eur. J. Nutr. 2020, 59, 3617–3634. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Xue, B.; Liu, Q.; Ou, S.; Wang, Y.; Peng, X. Different Flavonoids Can Shape Unique Gut Microbiota ProfileIn Vitro. J. Food Sci. 2016, 81, H2273–H2279. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Monici, K.D.S.; Costa, G.E.; Da Silva, N.; Reis, S.M.; De Oliveira, A.C. Bifidogenic effect of dietary fiber and resistant starch from leguminous on the intestinal microbiota of rats. Nutrition 2005, 21, 602–608. [Google Scholar] [CrossRef]
- Hara, H. Physiological Effects of Short-Chain Fatty Acid Produced from Prebiotics in the Colon. Biosci. Microflora 2002, 21, 35–42. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Yan, B.-B.; Xiao, Y.; Zhou, Y.-M.; Liu, T.-Y. Tartary buckwheat protein prevented dyslipidemia in high-fat diet-fed mice associated with gut microbiota changes. Food Chem. Toxicol. 2018, 119, 296–301. [Google Scholar] [CrossRef]
- Sánchez-Moya, T.; López-Nicolás, R.; Planes, D.; González-Bermúdez, C.A.; Ros-Berruezo, G.; Frontela-Saseta, C. In vitro modulation of gut microbiota by whey protein to preserve intestinal health. Food Funct. 2017, 8, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.-B.; Wen, J.; Zhao, Y.-C.; Tian, S.-J.; Zhang, X.-Y.; Wang, D.-H. Bifidobacterium pseudolongum reduces triglycerides by modulating gut microbiota in mice fed high-fat food. J. Steroid Biochem. Mol. Biol. 2020, 198, 105602. [Google Scholar] [CrossRef]
- Xie, M.; Chen, G.; Wan, P.; Dai, Z.; Zeng, X.; Sun, Y. Effects of Dicaffeoylquinic Acids from Ilex kudingcha on Lipid Metabolism and Intestinal Microbiota in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2019, 67, 171–183. [Google Scholar] [CrossRef]
- Si, X.; Shang, W.; Zhou, Z.; Strappe, P.; Wang, B.; Bird, A.; Blanchard, C. Gut Microbiome-Induced Shift of Acetate to Butyrate Positively Manages Dysbiosis in High Fat Diet. Mol. Nutr. Food Res. 2018, 62, 1700670. [Google Scholar] [CrossRef]
- De Silva, A.; Bloom, S.R. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver 2012, 6, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Pilon, G.; Dudonné, S.; Trottier, J.; St-Pierre, P.; Harris, C.S.; Lucas, M.; Lemire, M.; et al. Arctic berry extracts target the gut–liver axis to alleviate metabolic endotoxaemia, insulin resistance and hepatic steatosis in diet-induced obese mice. Diabetologia 2018, 61, 919–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Raychaudhuri, S.; Page, R.; Shahinozzaman; Obanda, D.N. Metagenomic insights into the effects of Urtica dioica vegetable on the gut microbiota of C57BL/6J obese mice, particularly the composition of Clostridia. J. Nutr. Biochem. 2021, 91, 108594. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef]
- Han, M.; Zhang, M.; Wang, X.; Bai, X.; Yue, T.; Gao, Z. Cloudy Apple Juice Fermented by Lactobacillus Prevents Obesity via Modulating Gut Microbiota and Protecting Intestinal Tract Health. Nutrients 2021, 13, 971. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Nielsen, D.S.G.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef] [Green Version]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from Different Geographic Origins Decreases Intestinal Inflammation and Bacteroides spp. Populations in a Model of DSS-Induced Colitis. Mol. Nutr. Food Res. 2018, 62, e1800080. [Google Scholar] [CrossRef]
- Kashtanova, D.A.; Tkacheva, O.N.; Doudinskaya, E.N.; Strazhesko, I.D.; Kotovskaya, Y.V.; Popenko, A.S.; Tyakht, A.V.; Alexeev, D.G. Gut Microbiota in Patients with Different Metabolic Statuses: Moscow Study. Microorganisms 2018, 6, 98. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.d.C.; Alfenas, R.d.C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, R.; Zhu, R.; Chen, B.; Tian, Y.; Zhang, H.; Xia, B.; Jia, Q.; Wang, L.; Zhao, D.; et al. Salvianolic acid B prevents body weight gain and regulates gut microbiota and LPS/TLR4 signaling pathway in high-fat diet-induced obese mice. Food Funct. 2020, 11, 8743–8756. [Google Scholar] [CrossRef]
- Tian, B.M.; Zhao, J.H.; Zhang, M.; Chen, Z.F.; Ma, Q.Y.; Liu, H.C.; Nie, C.X.; Zhang, Z.Q.; An, W.; Li, J.X. Lycium ruthenicum Anthocyanins Attenuate High-Fat Diet-Induced Colonic Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, 2000745. [Google Scholar] [CrossRef]
- Zhou, L.B.; Wang, X.; Shao, L.; Yang, Y.; Shang, W.B.; Yuan, G.Y.; Jiang, B.R.; Li, F.Y.; Tang, J.F.; Jing, H.; et al. Berberine acutely inhibits insulin secretion from beta-cells through 3′,5′-cyclic adenosine 5′-monophosphate signaling pathway. Endocrinology 2008, 149, 4510–4518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaumont, M.; Goodrich, J.K.; Jackson, M.; Yet, I.; Davenport, E.; Vieira-Silva, S.; Debelius, J.; Pallister, T.; Mangino, M.; Raes, J.; et al. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol. 2016, 17, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, P.; Zhang, Y.; Shou, Q.; Li, H.; Zhu, Y.; He, L.; Chen, J.; Jiao, J. Eicosapentaenoic and Docosahexaenoic Acids Differentially Alter Gut Microbiome and Reverse High-Fat Diet–Induced Insulin Resistance. Mol. Nutr. Food Res. 2020, 64, e1900946. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E.V. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177. [Google Scholar] [CrossRef]
- Ran, B.; Guo, C.; Li, W.; Li, W.; Wang, Q.; Qian, J.; Li, H. Sea buckthorn (Hippophae rhamnoides L.) fermentation liquid protects against alcoholic liver disease linked to regulation of liver metabolome and the abundance of gut microbiota. J. Sci. Food Agric. 2021, 101, 2846–2854. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-T.; Zhou, J.; Wu, C.-Y.; Zhang, W.; Shen, H.; Xu, J.-D.; Zhang, Y.-Q.; Long, F.; Li, S.-L. Protective effects of Poria cocos and its components against cisplatin-induced intestinal injury. J. Ethnopharmacol. 2021, 269, 113722. [Google Scholar] [CrossRef]
- Shao, X.; Sun, C.; Tang, X.; Zhang, X.; Han, D.; Liang, S.; Qu, R.; Hui, X.; Shan, Y.; Hu, L.; et al. Anti-Inflammatory and Intestinal Microbiota Modulation Properties of Jinxiang Garlic (Allium sativum L.) Polysaccharides toward Dextran Sodium Sulfate-Induced Colitis. J. Agric. Food Chem. 2020, 68, 12295–12309. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lei, S.; Liu, L.; Zhang, Y.; Zheng, B.; Zeng, H. Synergistic effect of lotus seed resistant starch and short-chain fatty acids on mice fecal microbiota in vitro. Int. J. Biol. Macromol. 2021, 183, 2272–2281. [Google Scholar] [CrossRef]
- Tanca, A.; Abbondio, M.; Palomba, A.; Fraumene, C.; Manghina, V.; Cucca, F.; Fiorillo, E.; Uzzau, S. Potential and active functions in the gut microbiota of a healthy human cohort. Microbiome 2017, 5, 1–15. [Google Scholar] [CrossRef]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Cho, K.Y. Altered Gut Microbiota and Shift in Bacteroidetes between Young Obese and Normal-Weight Korean Children: A Cross-Sectional Observational Study. BioMed Res. Int. 2020, 2020, 6587136. [Google Scholar] [CrossRef]
- Jorgensen, J.; Mortensen, P.B. Hydrogen sulfide and colonic epithelial metabolism—Implications for ulcerative colitis. Digest. Dis. Sci. 2001, 46, 1722–1732. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Appel, L.J.; Van Horn, L. Components of a Cardioprotective Diet New Insights. Circulation 2011, 123, 2870–2891. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Fang, Z.; Wahlqvist, M.L.; Wu, G.; Hodgson, J.M.; Johnson, S.K. Seed coats of pulses as a food ingredient: Characterization, processing, and applications. Trends Food Sci. Technol. 2018, 80, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Hou, D.; Fu, Y.; Xue, Y.; Guan, X.; Shen, Q. Adzuki Bean Alleviates Obesity and Insulin Resistance Induced by a High-Fat Diet and Modulates Gut Microbiota in Mice. Nutrients 2021, 13, 3240. https://doi.org/10.3390/nu13093240
Zhao Q, Hou D, Fu Y, Xue Y, Guan X, Shen Q. Adzuki Bean Alleviates Obesity and Insulin Resistance Induced by a High-Fat Diet and Modulates Gut Microbiota in Mice. Nutrients. 2021; 13(9):3240. https://doi.org/10.3390/nu13093240
Chicago/Turabian StyleZhao, Qingyu, Dianzhi Hou, Yongxia Fu, Yong Xue, Xiao Guan, and Qun Shen. 2021. "Adzuki Bean Alleviates Obesity and Insulin Resistance Induced by a High-Fat Diet and Modulates Gut Microbiota in Mice" Nutrients 13, no. 9: 3240. https://doi.org/10.3390/nu13093240
APA StyleZhao, Q., Hou, D., Fu, Y., Xue, Y., Guan, X., & Shen, Q. (2021). Adzuki Bean Alleviates Obesity and Insulin Resistance Induced by a High-Fat Diet and Modulates Gut Microbiota in Mice. Nutrients, 13(9), 3240. https://doi.org/10.3390/nu13093240