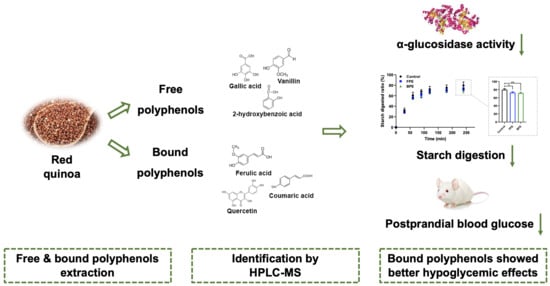
Bound Polyphenols from Red Quinoa Prevailed over Free Polyphenols in Reducing Postprandial Blood Glucose Rises by Inhibiting α-Glucosidase Activity and Starch Digestion
Abstract
:1. Introduction
2. Methods and Materials
2.1. Reagents and Materials
2.2. Extraction of FPE and BPE
2.3. Detection of Total Polyphenols Content and Total Flavonoids Content
2.4. Liquid Chromatography Analysis of FPE and BPE
2.5. DPPH and ABTS Assays
2.6. α-Glucosidase Inhibition Assay and Inhibition Kinetic Mode
2.7. Effects of FPE and BPE on the Digestion of Starch In Vitro
2.8. Effects of FPE and BPE on Postprandial Glucose in Mice
2.9. Statistical Analysis
3. Results
3.1. TPC, TFC and Antioxidant Activities of FPE and BPE
3.2. Components of FPE and BPE
3.3. Inhibitory Effects and Mechanism of FPE and BPE against α-Glucosidase
3.4. Effects of FPE and BPE on the Digestion of Rice Starch In Vitro
3.5. Effects of FPE and BPE on Postprandial Blood Glucose Levels In Vivo
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.; Liu, Y.; Zhan, L.; Rayat, G.R.; Xiao, J.; Jiang, H.; Li, X.; Chen, K. Anti-diabetic effects of natural antioxidants from fruits. Trends Food Sci. Technol. 2020, 117, 3–14. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, L.H.; Xie, J.H.; Liu, Y.; Chen, W. Pelargonidin-3-O-rutinoside as a novel alpha-glucosidase inhibitor for improving postprandial hyperglycemia. Chem. Commun. 2019, 55, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Choi, Y.H.; Cha, M.R.; Yoo, D.S.; Kim, Y.S.; Yon, G.H.; Hong, K.S.; Kim, Y.H.; Ryu, S.Y. Yeast alpha-Glucosidase Inhibition by Isoflavones from Plants of Leguminosae as an in Vitro Alternative to Acarbose. J. Agric. Food Chem. 2010, 58, 9988–9993. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Gidley, M.J.; Warren, F.J. The mechanism of interactions between tea polyphenols and porcine pancreatic alpha-amylase: Analysis by inhibition kinetics, fluorescence quenching, differential scanning calorimetry and isothermal titration calorimetry. Mol. Nutr. Food Res. 2017, 61, 1700324. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of alpha-amylase and alpha-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Zhang, X.W.; Dong, L.H.; Jia, X.C.; Liu, L.; Chi, J.W.; Huang, F.; Ma, Q.; Zhang, M.W.; Zhang, R.F. Bound Phenolics Ensure the Antihyperglycemic Effect of Rice Bran Dietary Fiber in db/db Mice via Activating the Insulin Signaling Pathway in Skeletal Muscle and Altering Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398. [Google Scholar] [CrossRef]
- Filho, A.M.; Pirozi, M.R.; Borges, J.T.; Sant’Ana, H.M.P.; Chaves, J.B.; Coimbra, J.S. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.H.; Chen, P.X.; Zhang, H.; Liu, R.H.; Tsao, R. Bound Phenolics of Quinoa Seeds Released by Acid, Alkaline, and Enzymatic Treatments and Their Antioxidant and alpha-Glucosidase and Pancreatic Lipase Inhibitory Effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef]
- Zhao, P.; Duan, L.; Guo, L.; Dou, L.L.; Dong, X.; Zhou, P.; Li, P.; Liu, E.H. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 2015, 173, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.S.; Cai, Y.Z.; Sun, M.; Wang, G.Y.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.; Chen, J.C.; Ye, X.Q.; Liu, D.H. In vitro inhibitory effects of Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves proanthocyanidins on pancreatic alpha-amylase and their interaction. Bioorg. Chem. 2020, 101, 104029. [Google Scholar] [CrossRef]
- Yan, S.S.; Shao, H.J.; Zhou, Z.H.; Wang, Q.; Zhao, L.H.; Yang, X.B. Non-extractable polyphenols of green tea and their antioxidant, anti-alpha-glucosidase capacity, and release during in vitro digestion. J. Funct. Foods 2018, 42, 129–136. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.H.; Zhang, B.; Chen, P.X.; Liu, R.H.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. Genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.Z.; Tao, W.Y.; Li, L.Q.; Wei, C.Y.; Duan, J.E.; Chen, S.G.; Ye, X.Q. Antioxidant and antiproliferative activities of proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves. J. Funct. Foods 2016, 27, 645–654. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Lucini, L. Gluten-free cereal-based food products: The potential of metabolomics to investigate changes in phenolics profile and their in vitro bioaccessibility. Curr. Opin. Food Sci. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J.M. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains-A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Antioxidant and alpha-glucosidase inhibitory activity of colored grains in China. J. Agric. Food Chem. 2010, 58, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Pinaffi, A.C.D.; Sampaio, G.R.; Soares, M.J.; Shahidi, F.; de Camargo, A.C.; Torres, E.A.F.S. Insoluble-Bound Polyphenols Released from Guarana Powder: Inhibition of Alpha-Glucosidase and Proanthocyanidin Profile. Molecules 2020, 25, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.Y.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with alpha-amylase and alpha-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhan, J.; Liu, X.L.; Wang, Y.; Ji, J.; He, Q.Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Oluwagbemigun, K.; Schmid, M.; Nothlings, U. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. Am. J. Clin. Nutr. 2018, 108, 49–61. [Google Scholar] [CrossRef] [Green Version]
- da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols Rich Diets and Risk of Type 2 Diabetes. Nutrients 2021, 13, 1445. [Google Scholar] [CrossRef]
- Wang, Y.; Alkhalidy, H.; Liu, D.M. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal. Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, S.; Xie, J.; Chen, Y.; Dong, R.; Zhang, X.; Liu, S.; Xie, J.; Hu, X.; Yu, Q. Antioxidant, α-amylase and α-glucosidase inhibitory activities of bound polyphenols extracted from mung bean skin dietary fiber. LWT 2020, 132, 109943. [Google Scholar] [CrossRef]
- Dong, R.H.; Liu, S.; Zheng, Y.T.; Zhang, X.J.; He, Z.C.; Wang, Z.P.; Wang, Y.Y.; Xie, J.H.; Chen, Y.; Yu, Q. Release and metabolism of bound polyphenols from carrot dietary fiber and their potential activity inin vitrodigestion and colonic fermentation. Food Funct. 2020, 11, 6652–6665. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Jiang, B.; Jiang, H.; Zhang, T.; Li, X.F. Interaction mechanism between green tea extract and human alpha-amylase for reducing starch digestion. Food Chem. 2015, 186, 20–25. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against alpha-amylase and alpha-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]





| Peak | Retention Time (min) | Formula | [M-H]− (m/z) | Identification | Content (mg/kg) | |
|---|---|---|---|---|---|---|
| FPE | BPE | |||||
| 1 | 5.84 | C7H6O3 | 137.03 | 2-hydroxybenzoic acid * | 4.927 ± 1.023 | ND |
| 2 | 6.22 | C8H8O3 | 151.04 | Vanillin | 3.170 ± 0.882 | ND |
| 3 | 8.04 | C9H8O4 | 179.04 | Caffeic acid | 2.388 ± 0.673 | ND |
| 4 | 10.84 | C7H6O5 | 169.01 | Gallic acid | 2.614 ± 0.541 | ND |
| 5 | 14.52 | C7H6O4 | 153.02 | 3,4-dihydroxy-benzoic acid | 2.774 ± 0.696 | 4.866 ± 0.334 |
| 6 | 15.98 | C8H8O4 | 167.04 | Vanillic acid | ND | 2.615 ± 0.272 |
| 7 | 19.98 | C15H14O6 | 289.07 | Catechin | ND | 2.788 ± 0.468 |
| 8 | 22.76 | C16H18O9 | 353.09 | Chlorogenic acid | ND | 3.387 ± 0.668 |
| 9 | 23.22 | C15H14O6 | 289.07 | Epicatechin | ND | 3.723 ± 0.334 |
| 10 | 26.46 | C9H8O3 | 163.04 | p-Coumaric acid | ND | 9.753 ± 0.268 |
| 11 | 27.66 | C10H10O4 | 193.05 | Ferulic acid | ND | 20.938 ± 3.866 |
| 12 | 28.44 | C16H20O9 | 355.02 | Ferulic acid 4-glucoside * | ND | 11.794 ± 1.225 |
| 13 | 29.32 | C15H10O7 | 301.04 | Quercetin | ND | 18.048 ± 1.236 |
| Samples | Double Reciprocal Equation | Km (mmol/L) | Vmax (OD/min) | R2 |
|---|---|---|---|---|
| No inhibitor | y = 28.046x + 6.602 | 4.248 | 0.151 | 0.9821 |
| FPE (20 mg/mL) | y = 46.893x + 11.507 | 4.075 | 0.087 | 0.9991 |
| BPE (20 mg/mL) | y = 26.946x + 15.011 | 1.795 | 0.067 | 0.9874 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Bai, B.; Yan, Y.; Liang, J.; Guan, X. Bound Polyphenols from Red Quinoa Prevailed over Free Polyphenols in Reducing Postprandial Blood Glucose Rises by Inhibiting α-Glucosidase Activity and Starch Digestion. Nutrients 2022, 14, 728. https://doi.org/10.3390/nu14040728
Zhang Y, Bai B, Yan Y, Liang J, Guan X. Bound Polyphenols from Red Quinoa Prevailed over Free Polyphenols in Reducing Postprandial Blood Glucose Rises by Inhibiting α-Glucosidase Activity and Starch Digestion. Nutrients. 2022; 14(4):728. https://doi.org/10.3390/nu14040728
Chicago/Turabian StyleZhang, Yu, Bing Bai, Yu Yan, Juan Liang, and Xiao Guan. 2022. "Bound Polyphenols from Red Quinoa Prevailed over Free Polyphenols in Reducing Postprandial Blood Glucose Rises by Inhibiting α-Glucosidase Activity and Starch Digestion" Nutrients 14, no. 4: 728. https://doi.org/10.3390/nu14040728
APA StyleZhang, Y., Bai, B., Yan, Y., Liang, J., & Guan, X. (2022). Bound Polyphenols from Red Quinoa Prevailed over Free Polyphenols in Reducing Postprandial Blood Glucose Rises by Inhibiting α-Glucosidase Activity and Starch Digestion. Nutrients, 14(4), 728. https://doi.org/10.3390/nu14040728