Intensive Medical Nutrition Therapy Alone or with Added Metformin to Prevent Gestational Diabetes Mellitus among High-Risk Mexican Women: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Intensive Medical Nutrition Therapy
2.4. Primary Endpoint
2.5. Secondary Outcomes
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. Women Included in the Study
3.2. Food Intake, Diet Adherence and Gestational Weight Gain
3.3. Incidence of Adverse Perinatal Outcomes
3.4. Adverse Effects to Metformin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Hartling, L.; Dryden, D.M.; Guthrie, A.; Muise, M.; Vandermeer, B.; Donovan, L. Benefits and Harms of Treating Gestational Diabetes Mellitus: A Systematic Review and Meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann. Intern. Med. 2013, 159, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martis, R.; Crowther, C.A.; Shepherd, E.; Alsweiler, J.; Downie, M.R.; Brown, J. Treatments for women with gestational diabetes mellitus: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2018, 2018, 1–109. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas Ninth edition 2019. In International Diabetes Federation, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: http://www.idf.org/about-diabetes/facts-figures (accessed on 5 January 2020).
- Reyes-Muñoz, E.; Parra, A.; Castillo-Mora, A.; Ortega-González, C. Effect of the Diagnostic Criteria of the International Association of Diabetes and Pregnancy Study Groups on the Prevalence of Gestational Diabetes Mellitus in Urban Mexican Women: A Cross-Sectional Study. Endocr. Pract. 2012, 18, 146–151. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin Number 190: Gestational Diabetes Mellitus Interim Update. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.M.; Simmons, D. Lessons learned from lifestyle prevention trials in gestational diabetes mellitus. Diabet. Med. 2019, 36, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trine Moholdt, T.; Hayman, M.; Shorakae, S.; Brown, W.J.; Harrison, C.L. The Role of Lifestyle Intervention in the Prevention and Treatment of Gestational Diabetes. Semin. Reprod. Med. 2020, 38, 398–406. [Google Scholar]
- Hyer, S.; Balani, J.; Shehata, H. Metformin in Pregnancy: Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2018, 19, 1954. [Google Scholar] [CrossRef] [Green Version]
- Griffith, R.J.; Alsweiler, J.; Moore, A.E.; Brown, S.; Middleton, P.; Shepherd, E.; Crowther, C.A. Interventions to prevent women from developing gestational diabetes mellitus: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020, 6, CD012394. [Google Scholar] [PubMed]
- Reyes-Muñoz, E.; Martínez-Herrera, E.M.; Ortega-González, C.; Arce-Sánchez, L.; Ávila-Carrasco, A.; Zamora-Escudero, R. HOMA-IR and QUICKI reference values during pregnancy in Mexican women. Ginecol. Obstet. Mex. 2017, 85, 306–313. [Google Scholar]
- Simmons, D. Safety Considerations with Pharmacological Treatment of Gestational Diabetes Mellitus. Drug Saf. 2015, 38, 65–78. [Google Scholar] [CrossRef]
- Martínez-Cruz, N.; Rapisarda, A.G.C.; Soriano-Ortega, K.P.; Arce-Sánchez, L.; Cianci, A.; Ortega-Gonzalez, C.; Torres-Herrera, U.; Espino-y-Sosa, S.; Estrada-Gutierrez, G.; Montoya-Estrada, A.; et al. Perinatal Outcomes in Mexican Women with Untreated Mild Gestational Diabetes Mellitus Diagnosed by the International Association of Diabetes and Pregnancy Study Groups Criteria. Diabetes Metab. Syndr. Obes. 2019, 12, 2667–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mexican Secretary of Health. Diabetes and Pregnancy, Technical Guideline. Mexico, Secretary of Health. 2017. Available online: http://cnegsr.salud.gob.mx/contenidos/descargas/SMP/LineamientoDiabetesyEmbarazo.pdf (accessed on 25 July 2020).
- American Diabetes Association. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. S1), S183–S192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Lowe, L.P.; Dyer, A.R.; Oats, J.J.N.; Buchanan, T.A.; International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 159: Management of Preterm Labor. Obstet. Gynecol. 2016, 127, e29–e38. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.I.; Butt, K.; Naud, K.; Smithies, M. Amniotic Fluid: Technical Update on Physiology and Measurement. Obstet. Gynaecol. Can. 2017, 39, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Huerta, S.F.; Salgado, H.M. Birth weight of male and female infants born in hospitals affiliated with the Instituto Mexicano del Seguro Social. Bol. Med. Hosp. Infant. Mex. 2012, 69, 30–39. [Google Scholar]
- Glueck, C.J.; Wang, P.; Kobayashi, S.; Phillips, H.; Sieve-Smith, L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil. Steril. 2002, 77, 520–525. [Google Scholar] [CrossRef]
- Kenneth, F.; Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar]
- Morris, S.F.; Wylie-Rosett, J. Medical Nutrition Therapy: A Key to Diabetes Management and Prevention. Clin. Diabetes 2010, 28, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cruz, N.; Rapisarda, A.M.C.; Soriano-Ortega, K.P.; Arce-Sánchez, L.; Cianci, A.; Ortega-Gonzalez, C.; Torres-Herrera, U.; Espino-y-Sosa, S.; Estrada-Gutierrez, G.; Montoya-Estrada, A. Diet and Nutritional Interventions with the Special Role of Myo-Inositol in Gestational Diabetes Mellitus Management. An Evidence-Based Critical Appraisal. Curr. Pharm. Des. 2019, 25, 2467–2473. [Google Scholar]
- Bennett, C.J.; Walker, R.E.; Blumfield, M.L.; Gwini, S.-M.; Ma, J.; Wang, F.; Wan, Y.; Dickinson, H.; Truby, H. Interventions designed to reduce excessive gestational weight gain can reduce the incidence of gestational diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabetes Res. Clin. Pract. 2018, 141, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, E.; Gomersall, J.C.; Tieu, J.; Han, S.; Crowther, C.A.; Middleton, P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2017, 11, CD010443. [Google Scholar] [CrossRef]
- Reyes-Muñoz, E.; Guardo, F.D.; Ciebiera, M.; Kahramanoglu, I.; Sathyapalan, T.; Lin, L.; Shah, M.; Karaman, E.; Fan, S.; Zito, G. Use of myo-inositol plus Bifidobacterium lactis and Lactobacillus rhamnosus for preventing gestational diabetes mellitus in Mexican women. Gac. Med. Mex. 2020, 156 (Suppl. S3), S51–S57. [Google Scholar] [PubMed]
- Chatzakis, C.; Goulis, D.G.; Mareti, E.; Eleftheriades, M.; Zavlanos, A.; Dinas, K.; Sotiriadis, A. Prevention of gestational diabetes mellitus in overweight or obese pregnant women: A network meta-analysis. Diabetes Res. Clin. Pract. 2019, 158, 107924. [Google Scholar] [CrossRef] [PubMed]
- Chiswick, C.; Reynolds, R.; Denison, F.; Drake, A.J.; Forbes, S.; Newby, D.E.; Walker, B.R.; Quenby, S.; Wray, S.; Weeks, A.; et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Dodd, J.M.; Louise, J.; Deussen, A.R.; Grivell, R.; Dekker, G.; McPhee, A.J.; Hague, W. Effect of metformin in addition to dietary and lifestyle advice for pregnant women who are overweight or obese: The GRoW randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 15–24. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]

| Characteristics | Women Who Declined Participation (n = 110) | Women with Initial OGTT (n = 131) | p Value * |
|---|---|---|---|
| Maternal age (years) | 31.9 ± 5 | 32.3 ± 5 | 0.59 |
| Pregestational weight (kg) | 72.8 ± 10.1 | 74.0 ± 12.2 | 0.44 |
| Pregestational BMI (kg/m2) | 30.0 ± 3.8 | 30.2± 4.4 | 0.76 |
| Number of previous gestations | 2.6 ± 1.2 | 2.5 ± 1.3 | 0.71 |
| Weeks of gestation | 13.4 ± 0.8 | 13.4 ± 1.3 | 0.99 |
| 75 g-2 h OGTT | |||
| Fasting (mg/mL) | - | 84.6 ± 8.7 | - |
| 1 h (mg/mL) | - | 129.5 ± 28.4 | - |
| 2 h (mg/mL) | - | 119.1 ± 21.2 | - |
| Latino ethnic group | 110 (100) | 131 (100) | 0.98 |
| Maternal age > 35 years | 37 (33.6) | 47 (35.8) | 0.82 |
| Overweight (pBMI 25–29.9 kg/m2) | 49 (44.5) | 56 (42.7) | 0.88 |
| Obesity (pBMI ≥ 30 kg/m2) | 51 (46.4) | 65 (49.6) | 0.71 |
| Insulin resistance (HOMA-IR > 2.5) | - | 66 (50.3) | - |
| History of GDM | 1 (0.9) | 1 (0.8) | 0.91 |
| History of Macrosomia | 4 (3.6) | 5 (3.8) | 0.76 |
| History of PCOS | 12 (10.9) | 16 (12.2) | 0.70 |
| History of prediabetes | 17 (15.4) | 22 (16.7) | 0.91 |
| History of Infertility | 61 (55.5) | 74 (56.5) | 0.87 |
| First degree relative with DM | 70 (63.6) | 91 (69.4) | 0.41 |
| Characteristics | Women Who Declined Enrollment (n = 41) | Women Randomized at the Study (n = 90) | p Value * |
|---|---|---|---|
| Maternal age (years) | 31.5 ± 6.1 | 32.6 ± 4.9 | 0.29 |
| Pregestational weight (kg) | 72.7 ± 11.1 | 74.5 ± 12.7 | 0.43 |
| Pregestational BMI (kg/m2) | 30.1 ± 3.9 | 30.2 ± 4.6 | 0.81 |
| Number of previous gestations | 2.5 ± 1.4 | 2.4 ± 1.2 | 0.53 |
| Weeks of gestation | 13.4 ± 0.68 | 13.4 ± 1.5 | 0.92 |
| 75 g-2 h OGTT | |||
| Fasting (mg/mL) | 85.8 ± 9.0 | 83.5 ± 8.2 | 0.15 |
| 1 h (mg/mL) | 124.3 ± 32 | 129.3 ± 26.7 | 0.34 |
| 2 h (mg/mL) | 113.6 ± 22.2 | 114.1 ± 20.8 | 0.90 |
| Latino ethnic group | 41 (100) | 90 (100) | 0.98 |
| Maternal age > 35 years | 14 (34.1) | 33 (36.6) | 0.53 |
| Overweight (pBMI 25–29.9 kg/m2) | 17 (41.4) | 39 (43.3) | 0.99 |
| Obesity (pBMI ≥ 30 kg/m2) | 21 (51.2) | 44 (48.8) | 0.95 |
| Insulin resistance (HOMA-IR > 2.5) | 19 (46.3) | 47 (52.2) | 0.66 |
| History of GDM | 0 (0) | 1 (1.1) | 0.57 |
| History of Macrosomia | 1 (2.4) | 4 (4.4) | 0.94 |
| History of PCOS | 6 (14.6) | 10 (11.1) | 0.78 |
| History of prediabetes | 6 (14.6) | 16 (17.7) | 0.84 |
| History of Infertility | 25 (60.9) | 49 (54.4) | 0.61 |
| First degree relative with DM | 27 (65.8) | 64 (71.1) | 0.89 |
| Characteristics | Group 1 MNT + Metformin (n = 45) | Group 2 MNT (n = 45) | p Value * |
|---|---|---|---|
| Maternal age (years) | 32.4 ± 5.1 | 32.8 ± 4.7 | 0.68 |
| Pregestational weight (kg) | 73.6 ± 13.9 | 75.49 ±11.5 | 0.50 |
| Pregestational BMI (Kg/m2) | 30.03 ± 5.1 | 30.45 ± 4.0 | 0.67 |
| Number of previous gestations | 2.3 ± 1.1 | 2.6 ± 1.5 | 0.56 |
| Weeks of gestation | 13.3 ± 1.5 | 13.6 ± 1.5 | 0.40 |
| Total energy intake (Kcal/day) | 1922 ± 600 | 1995 ± 722 | 0.64 |
| Carbohydrates intake (g/day) | 268 ± 97 | 279 ± 102 | 0.62 |
| Fasting insulin (μU/mL) | 13.9 ± 9.2 | 13.2 ± 8.5 | 0.71 |
| HOMA-IR | 2.8 ± 1.8 | 2.7 ± 1.7 | 0.75 |
| 75 g-2 h OGTT | |||
| Fasting (mg/mL) | 83.3 ± 8.6 | 83.7 ± 7.9 | 0.82 |
| 1 h (mg/mL) | 129.5 ± 27.6 | 130.2 ± 25.5 | 0.90 |
| 2 h (mg/mL) | 111.3 ± 19.8 | 117 ± 21.2 | 0.19 |
| Characteristics | Group 1: MNT + Metformin n = 45 (%) | Group 2: MNT n = 45 (%) | p Value * |
|---|---|---|---|
| Risk factors | |||
| Latino ethnic group | 45 (100) | 45 (100) | 0.98 |
| Maternal age > 35 years | 17 (37.8) | 16 (35.6) | 0.83 |
| Overweight (pBMI 25–29.9 kg/m2) | 18 (40) | 21 (46.7) | 0.67 |
| Obesity (pBMI ≥ 30 kg/m2) | 22 (48.9) | 22 (48.9) | 0.83 |
| Insulin resistance (HOMA >2.5) | 26 (57.8) | 21 (46.7) | 0.29 |
| History of GDM | 1 (2.2) | 0 (0.0) | 0.32 |
| History of Macrosomia | 1 (2.2) | 3 (6.6) | 0.29 |
| History of PCOS | 5 (11.1) | 5 (11.1) | 0.96 |
| History of prediabetes | 9 (20) | 7 (15.5) | 0.54 |
| History of Infertility | 21 (46.6) | 28 (62.2) | 0.13 |
| First degree relative with DM | 34 (75.5) | 30 (66.6) | 0.43 |
| Comorbidities | |||
| Leiomiomas diameter < 3 cm | 6 (13.3) | 3 (6.7) | 0.29 |
| Hypothyroidism | 12 (26.6) | 14 (31.1) | 0.64 |
| Asthma | 2 (4.4) | 1 (2.2) | 0.55 |
| Cervical incompetence | 4 (8.9) | 4 (8.9) | 0.98 |
| Food Component | Group 1 MNT + Metformin n = 45 (%) | Group 2 MNT n = 45 (%) | p Value * |
|---|---|---|---|
| Energy (Kcal/day) | 1804 ± 639 | 1908 ± 496 | 0.43 |
| Carbohydrates (g/day) | 237 ± 89 | 260 ±85 | 0.24 |
| Dietary fiber (g/day) | 26.7 ± 11 | 30 ± 12 | 0.21 |
| Carbohydrates (%) | 52.6 ± 6.6 | 54.6 ± 8.1 | 0.23 |
| Fat (%) | 29.4 ± 6.3 | 28.2 ± 7.2 | 0.43 |
| Outcome | Group 1 MNT + Metformin n = 45 (%) | Group 2 MNT n = 45 (%) | Relative Risk (95% CI) |
|---|---|---|---|
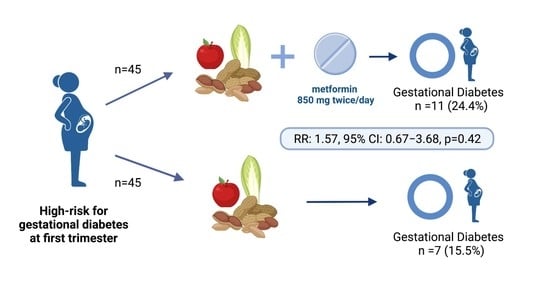
| Gestational diabetes mellitus | 11 (24.4) | 7 (15.5) | 1.57 (0.67–3.68) |
| Preeclampsia | 2 (4.4) | 4 (8.8) | 0.46 (0.9–2.4) |
| Gestational hypertension | 5 (11.1) | 1 (2.2) | 5.6 (0.7–44.5) |
| Polyhydramnios | 1 (2.2) | 3 (6.7) | 0.31 (0.03–2.8) |
| Oligohydramnios | 3 (6.7) | 3 (6.7) | 0.93 (0.19–4.3) |
| Preterm birth | 5 (11.1) | 8 (17.8) | 0.58 (0.2–1.6) |
| Caesarean section | 31 (68.9) | 35 (77.8) | 0.88 (0.7–1.1) |
| Congenital malformations | 0 (0.0) | 3 (6.7) | 0.25 (0.02–2.1) |
| Characteristics | Group 1 MNT + Metformin n = 43 | Group 2 MNT n = 43 | p |
|---|---|---|---|
| Weeks of gestation | 37.3 ± 3.8 | 37.5 ± 2.5 | 0.71 |
| Length (cm) | 48.28 ± 2.6 | 47.97 ± 3.5 | 0.65 |
| Weight (g) | 2872.8 ± 504 | 2840.7 ± 556 | 0.78 |
| Large for gestational age (n,%) | 1 (2.3) | 2 (4.6) | 0.52 |
| Small for gestational age (n,%) | 6 (13.9) | 6 (13.9) | 0.92 |
| Admission to NICU (n,%) | 0 (0.0) | 1 (2.3) | 0.98 |
| Admission to NIMCU (n,%) | 11 (25.6) | 9 (20.9) | 0.79 |
| Death (n,%) | 1 (2.3) | 1 (2.3) | 0.98 |
| Adverse Effect | Group 1 MNT + Metformin n= 45 (%) | Group 2 MNT n = 45 (%) | Relative Risk (95% CI) |
|---|---|---|---|
| Headache | 3 (6.6) | 2 (4.4) | 1.5 (0.27–8.7) |
| Heartburn | 15 (33.3) | 11 (24.4) | 1.4 (0.73–2.6) |
| Dyspepsia | 8 (17.8) | 12 (26.7) | 0.66 (0.30–1.47) |
| Diarrhea | 2 (4.4) | 0 (0.0) | 3 (0.32–27.7) |
| Constipation | 5 (11.1) | 8 (17.8) | 0.64 (0.23–1.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perichart-Perera, O.; Mier-Cabrera, J.; Flores-Robles, C.M.; Martínez-Cruz, N.; Arce-Sánchez, L.; Alvarado-Maldonado, I.N.; Montoya-Estrada, A.; Romo-Yañez, J.; Rodríguez-Cano, A.M.; Estrada-Gutierrez, G.; et al. Intensive Medical Nutrition Therapy Alone or with Added Metformin to Prevent Gestational Diabetes Mellitus among High-Risk Mexican Women: A Randomized Clinical Trial. Nutrients 2022, 14, 62. https://doi.org/10.3390/nu14010062
Perichart-Perera O, Mier-Cabrera J, Flores-Robles CM, Martínez-Cruz N, Arce-Sánchez L, Alvarado-Maldonado IN, Montoya-Estrada A, Romo-Yañez J, Rodríguez-Cano AM, Estrada-Gutierrez G, et al. Intensive Medical Nutrition Therapy Alone or with Added Metformin to Prevent Gestational Diabetes Mellitus among High-Risk Mexican Women: A Randomized Clinical Trial. Nutrients. 2022; 14(1):62. https://doi.org/10.3390/nu14010062
Chicago/Turabian StylePerichart-Perera, Otilia, Jennifer Mier-Cabrera, Claudia Montserrat Flores-Robles, Nayeli Martínez-Cruz, Lidia Arce-Sánchez, Itzel Nallely Alvarado-Maldonado, Araceli Montoya-Estrada, José Romo-Yañez, Ameyalli Mariana Rodríguez-Cano, Guadalupe Estrada-Gutierrez, and et al. 2022. "Intensive Medical Nutrition Therapy Alone or with Added Metformin to Prevent Gestational Diabetes Mellitus among High-Risk Mexican Women: A Randomized Clinical Trial" Nutrients 14, no. 1: 62. https://doi.org/10.3390/nu14010062
APA StylePerichart-Perera, O., Mier-Cabrera, J., Flores-Robles, C. M., Martínez-Cruz, N., Arce-Sánchez, L., Alvarado-Maldonado, I. N., Montoya-Estrada, A., Romo-Yañez, J., Rodríguez-Cano, A. M., Estrada-Gutierrez, G., Espino y Sosa, S., Guzmán-Huerta, M., Ayala-Yañez, R., & Reyes-Muñoz, E. (2022). Intensive Medical Nutrition Therapy Alone or with Added Metformin to Prevent Gestational Diabetes Mellitus among High-Risk Mexican Women: A Randomized Clinical Trial. Nutrients, 14(1), 62. https://doi.org/10.3390/nu14010062