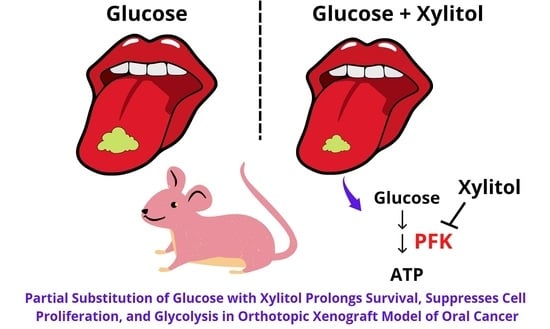
Partial Substitution of Glucose with Xylitol Prolongs Survival and Suppresses Cell Proliferation and Glycolysis of Mice Bearing Orthotopic Xenograft of Oral Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size and Power Calculation
2.2. Animals and Maintenance
2.3. Orthotopic Tongue Cancer Xenograft Model
2.4. Interventions
2.5. Outcome Measurement
2.6. Histopathological Analysis
2.7. Immunohistochemical Analyses
2.8. Phosphofructokinase Enzyme Activity
2.9. Statistical Analysis
3. Results
3.1. Establishment of Orthotopic Tongue Cancer Xenograft Model
3.2. No Significant Differences in Body Weights and No Adverse Effects in Weight Loss of Xylitol-Treated Mice, Compared to the Control Group
3.3. No Significant Differences in Diet, Water, and Hydrogel Intakes of Xylitol-Treated Mice, Compared to the Control Group
3.4. Non-Significant Reduction in Tumor Volumes of Xylitol-Treated Mice, Compared to Control Group
3.5. Significant Prolongation of Survival in Xylitol-Treated Mice, Compared to Control Group
3.6. Significant Decrease in the Intense-to-Mild Staining Ratio of Proliferation Marker Ki-67 in Tongue Tumor of Xylitol-Treated Mice, Compared to Control Group
3.7. Significant Suppression in the Rate-Limiting Glycolytic Enzyme PFK in Tongue Tumors of Xylitol-Treated Mice, Compared to Control Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Insamran, W.; Sangrajrang, S. National Cancer Control Program of Thailand. Asian Pac. J. Cancer Prev. 2020, 21, 577–582. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. 2020. Available online: https://gco.iarc.fr/today (accessed on 23 February 2022).
- Ren, Z.H.; Hu, C.Y.; He, H.R.; Li, Y.J.; Lyu, J. Global and regional burdens of oral cancer from 1990 to 2017: Results from the global burden of disease study. Cancer Commun. 2020, 40, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, M.; Riera March, A. Tongue Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sierpina, V.; Levine, L.; McKee, J.; Campbell, C.; Lian, S.; Frenkel, M. Nutrition, metabolism, and integrative approaches in cancer survivors. Semin. Oncol. Nurs. 2015, 31, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Chingsuwanrote, P.; Yoosadiang, P.; Mekkriangkrai, D.; Ratchawong, T.; Buraphacheep, N.; Kijanukul, S.; Saekhow, S.; Pongpitchayadej, O.; Vongvachvasin, K.; et al. Partial Substitution of Glucose with Xylitol Suppressed the Glycolysis and Selectively Inhibited the Proliferation of Oral Cancer Cells. Nutr. Cancer 2017, 69, 862–872. [Google Scholar] [CrossRef]
- Di Maso, M.; Dal Maso, L.; Augustin, L.S.A.; Puppo, A.; Falcini, F.; Stocco, C.; Mattioli, V.; Serraino, D.; Polesel, J. Adherence to the Mediterranean Diet and Mortality after Breast Cancer. Nutrients 2020, 12, 3649. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [Green Version]
- Milenkovic, T.; Bozhinovska, N.; Macut, D.; Bjekic-Macut, J.; Rahelic, D.; Velija Asimi, Z.; Burekovic, A. Mediterranean Diet and Type 2 Diabetes Mellitus: A Perpetual Inspiration for the Scientific World. A Review. Nutrients 2021, 13, 1307. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Annibaldi, A.; Widmann, C. Glucose metabolism in cancer cells. Curr. Opin. Clin. Nutr. Metab Care 2010, 13, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, N.; Ahmed, K.B.; Garcia-Prieto, C.; Huang, P. Metabolic alterations in cancer cells and therapeutic implications. Chin. J. Cancer 2011, 30, 508–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, V.; Macho, M.; Ewe, D.; Singh, M.; Saha, S.; Saurav, K. Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism. Foods 2020, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Natah, S.S.; Hussien, K.R.; Tuominen, J.A.; Koivisto, V.A. Metabolic response to lactitol and xylitol in healthy men. Am. J. Clin. Nutr. 1997, 65, 947–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef]
- Yalcin, A.; Telang, S.; Clem, B.; Chesney, J. Joint FAO/WHO Food Standards Programme. Codex Alimentarius Commission Twenty-First Session; WHO Food Additives Series No. 12; Joint FAO/WHO Codex Alimentarius Commission: London, UK, 1995. [Google Scholar]
- Federation of American Societies for Experimental Biology (FASEB). The Evaluation of the Energy of Certain Sugar Alcohols Used as Food Ingredients; Federation of American Societies for Experimental Biology (FASEB): Bethesda, MD, USA, 1994. [Google Scholar]
- Janakiram, C.; Deepan Kumar, C.V.; Joseph, J. Xylitol in preventing dental caries: A systematic review and meta-analyses. J. Nat. Sci. Biol. Med. 2017, 8, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Mota, K.R.; da Silva, J.V.F.; Borges, C.D.; Leite de Marcelos, P.G.C.; Alvares, P.R.; Santos Júnior, V.E.D. Effectiveness of the use of xylitol chewing gum in prevention of dental caries: A systematic review. J. Indian Soc. Pedod. Prev. Dent. 2021, 39, 113–119. [Google Scholar] [CrossRef]
- Park, E.; Park, M.H.; Na, H.S.; Chung, J. Xylitol induces cell death in lung cancer A549 cells by autophagy. Biotechnol. Lett. 2015, 37, 983–990. [Google Scholar] [CrossRef]
- Park, E.; Na, H.S.; Kim, S.M.; Wallet, S.; Cha, S.; Chung, J. Xylitol, an anticaries agent, exhibits potent inhibition of inflammatory responses in human THP-1-derived macrophages infected with Porphyromonas gingivalis. J. Periodontol. 2014, 85, e212–e223. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Sumardika, I.W.; Saito, S.; Ruma, I.M.W.; Kondo, E.; Shibukawa, M.; Sakaguchi, M. Identification of a novel component leading to anti-tumor activity besides the major ingredient cordycepin in Cordyceps militaris extract. J. Chromatogr. B 2017, 1061–1062, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Tomonobu, N.; Komalasari, N.L.G.Y.; Sumardika, I.W.; Jiang, F.; Chen, Y.; Yamamoto, K.-i.; Kinoshita, R.; Murata, H.; Inoue, Y.; Sakaguchi, M. Xylitol acts as an anticancer monosaccharide to induce selective cancer death via regulation of the glutathione level. Chem.-Biol. Interact. 2020, 324, 109085. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.N.; Holsinger, F.C.; Jasser, S.A.; Bekele, B.N.; Fidler, I.J. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin. Cancer Res. 2002, 8, 293–298. [Google Scholar] [PubMed]
- Sano, D.; Myers, J.N. Xenograft models of head and neck cancers. Head Neck Oncol. 2009, 1, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, D.; Xie, T.X.; Ow, T.J.; Zhao, M.; Pickering, C.R.; Zhou, G.; Sandulache, V.C.; Wheeler, D.A.; Gibbs, R.A.; Caulin, C.; et al. Disruptive TP53 mutation is associated with aggressive disease characteristics in an orthotopic murine model of oral tongue cancer. Clin. Cancer Res. 2011, 17, 6658–6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.H.; Kim, W.Y.; Lee, O.H.; Kang, J.H.; Woo, J.K.; Kim, J.H.; Glisson, B.; Lee, H.Y. Insulin-like growth factor binding protein-3 suppresses vascular endothelial growth factor expression and tumor angiogenesis in head and neck squamous cell carcinoma. Cancer Sci. 2012, 103, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Haboubi, N. Pathology and Genetics: Tumours of the Digestive System; Hamilton, S.R., Aaltonen, L.A., Eds.; IARC Press: Lyon, France, 2000; Volume 9, pp. 144–145. ISBN 02-832-2410-8. [Google Scholar]
- Lam-Ubol, A.; Fitzgerald, A.L.; Myers, J.N.; Trachootham, D. Histopathologic Characters of Tumor Specimens from Oral Cancer Xenograft in Nude Mice Treated With Beta-Phenethyl Isothiocyanate (PEITC). Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, e157. [Google Scholar] [CrossRef]
- Grimm, M.; Cetindis, M.; Lehmann, M.; Biegner, T.; Munz, A.; Teriete, P.; Kraut, W.; Reinert, S. Association of cancer metabolism-related proteins with oral carcinogenesis—Indications for chemoprevention and metabolic sensitizing of oral squamous cell carcinoma? J. Transl. Med. 2014, 12, 208. [Google Scholar] [CrossRef] [Green Version]
- Twetman, S. Consistent evidence to support the use of xylitol- and sorbitol-containing chewing gum to prevent dental caries. Evid. Based Dent. 2009, 10, 10–11. [Google Scholar] [CrossRef] [Green Version]
- Ortega, Á.D.; Sánchez-Aragó, M.; Giner-Sánchez, D.; Sánchez-Cenizo, L.; Willers, I.; Cuezva, J.M. Glucose avidity of carcinomas. Cancer Lett. 2009, 276, 125–135. [Google Scholar] [CrossRef]
- Salli, K.; Lehtinen, M.J.; Tiihonen, K.; Ouwehand, A.C. Xylitol’s Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients 2019, 11, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyasawa, H.; Iwami, Y.; Mayanagi, H.; Takahashi, N. Xylitol inhibition of anaerobic acid production by Streptococcus mutans at various pH levels. Oral Microbiol. Immunol. 2003, 18, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.A.; Nayak, U.A.; Khandelwal, V. The effect of xylitol on dental caries and oral flora. Clin. Cosmet. Investig. Dent. 2014, 6, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulukutla, B.C.; Yongky, A.; Le, T.; Mashek, D.G.; Hu, W.S. Regulation of Glucose Metabolism—A Perspective From Cell Bioprocessing. Trends Biotechnol. 2016, 34, 638–651. [Google Scholar] [CrossRef]
- Tennant, D.R. Potential intakes of total polyols based on UK usage survey data. Food Addit. Contaminants. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Storey, D.; Lee, A.; Bornet, F.; Brouns, F. Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid. Eur. J. Clin. Nutr. 2007, 61, 349–354. [Google Scholar] [CrossRef]
- Mäkinen, K.K. Gastrointestinal Disturbances Associated with the Consumption of Sugar Alcohols with Special Consideration of Xylitol: Scientific Review and Instructions for Dentists and Other Health-Care Professionals. Int. J. Dent. 2016, 2016, 5967907. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, M.; Qi, M.; Tian, Z.; Wu, W.; Yang, J.; Zhang, M.; Tang, L.; Tang, X. The antilymphatic metastatic effect of hyaluronic acid in a mouse model of oral squamous cell carcinoma. Cancer Biol. Ther. 2020, 21, 541–548. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]









| Groups | Glucose (g/kg Body Weight) | Xylitol (g/kg Body Weight) | Energy input (kcal/kg Body Weight) |
|---|---|---|---|
| Control group | 1.59 | - | 6.4 |
| Experimental group 1 | Low glucose (0.97) | 1.03 1 | 6.4 |
| Experimental group 2 | Low glucose (0.35) | 2.06 1 | 6.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahasakul, Y.; Angkhasirisap, W.; Lam-ubol, A.; Aursalung, A.; Sano, D.; Takada, K.; Trachootham, D. Partial Substitution of Glucose with Xylitol Prolongs Survival and Suppresses Cell Proliferation and Glycolysis of Mice Bearing Orthotopic Xenograft of Oral Cancer. Nutrients 2022, 14, 2023. https://doi.org/10.3390/nu14102023
Sahasakul Y, Angkhasirisap W, Lam-ubol A, Aursalung A, Sano D, Takada K, Trachootham D. Partial Substitution of Glucose with Xylitol Prolongs Survival and Suppresses Cell Proliferation and Glycolysis of Mice Bearing Orthotopic Xenograft of Oral Cancer. Nutrients. 2022; 14(10):2023. https://doi.org/10.3390/nu14102023
Chicago/Turabian StyleSahasakul, Yuraporn, Wannee Angkhasirisap, Aroonwan Lam-ubol, Amornrat Aursalung, Daisuke Sano, Kentaro Takada, and Dunyaporn Trachootham. 2022. "Partial Substitution of Glucose with Xylitol Prolongs Survival and Suppresses Cell Proliferation and Glycolysis of Mice Bearing Orthotopic Xenograft of Oral Cancer" Nutrients 14, no. 10: 2023. https://doi.org/10.3390/nu14102023
APA StyleSahasakul, Y., Angkhasirisap, W., Lam-ubol, A., Aursalung, A., Sano, D., Takada, K., & Trachootham, D. (2022). Partial Substitution of Glucose with Xylitol Prolongs Survival and Suppresses Cell Proliferation and Glycolysis of Mice Bearing Orthotopic Xenograft of Oral Cancer. Nutrients, 14(10), 2023. https://doi.org/10.3390/nu14102023