Selenium Yeast and Fish Oil Combination Diminishes Cancer Stem Cell Traits and Reverses Cisplatin Resistance in A549 Sphere Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents and Chemicals
2.3. Images of the Cells
2.4. Colony Formation Assay
2.5. Side Population Detection by DyeCycle Violet (DCV) Exclusion
2.6. Apoptotic and Necrotic Cell Death Detected by Annexin V/7-Amino-Actinomycin D Double Staining
2.7. Measurement of Apoptotic Sub-G1 Fraction
2.8. Western Blot
2.9. Antibodies
2.10. Cell Viability Measurement
2.11. Analysis of Synergistic Combination Effect
3. Results
3.1. CSC-like A549 Sphere Cells Possessed Elevated GRP78 and Reduced CHOP and AMPK Activity
3.2. Se-Y and FO Synergistically Induced Apoptosis of A549 Sphere Cells and Diminished CSC Traits
3.3. Se-Y and FO Combination Suppressed the Side Population of A549 Sphere Cells Via AMPK Activation
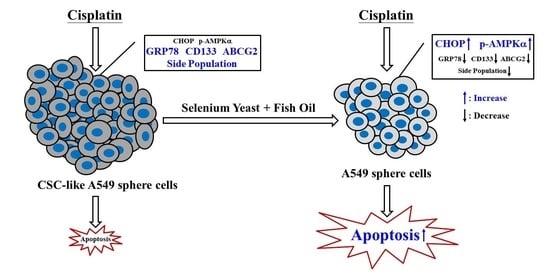
3.4. Se-Y and FO Combination Reversed Cisplatin Resistance in A549 Sphere Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Dericks, L.; Galetta, D. Impact of Cancer Stem Cells and Cancer Stem Cell-Driven Drug Resiliency in Lung Tumor: Options in Sight. Cancers 2022, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Soerjomataram, I.; Ferlay, J.; Rutherford, M.; Weiderpass, E.; Bray, F. International trends in lung cancer incidence by histological subtype: Adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 2014, 84, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gairola, K.; Gururani, S.; Bahuguna, A.; Garia, V.; Pujari, R.; Dubey, S.K. Natural products targeting cancer stem cells: Implications for cancer chemoprevention and therapeutics. J. Food Biochem. 2021, 45, e13772. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Yang, C.-J.; Huang, M.-S.; Yeh, C.-T.; Wu, A.T.; Lee, Y.-C.; Lai, T.-C.; Lee, C.-H.; Hsiao, Y.-W.; Lu, J.; et al. Cisplatin Selects for Multidrug-Resistant CD133+ Cells in Lung Adenocarcinoma by Activating Notch Signaling. Cancer Res. 2013, 73, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, G.; Roz, L.; Perego, P.; Tortoreto, M.; Fontanella, E.; Gatti, L.; Pratesi, G.; Fabbri, A.; Andriani, F.; Tinelli, S.; et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc. Natl. Acad. Sci. USA 2009, 106, 16281–16286. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.-P.; Liao, J.; Tang, Z.-J.; Wu, W.-J.; Yang, J.; Zeng, Z.-L.; Hu, Y.; Wang, P.; Ju, H.-Q.; Xu, R.-H.; et al. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ. 2013, 21, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Moselhy, J.; Srinivasan, S.; Ankem, M.K.; Damodaran, C. Natural Products That Target Cancer Stem Cells. Anticancer Res. 2015, 35, 5773–5788. [Google Scholar]
- Burnett, J.; Newman, B.; Sun, D. Targeting cancer stem cells with natural products. Curr. Drug Targets 2012, 13, 1054–1064. [Google Scholar] [CrossRef]
- Scarpa, E.-S.; Ninfali, P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int. J. Mol. Sci. 2015, 16, 15727–15742. [Google Scholar] [CrossRef] [Green Version]
- Naujokat, C. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, V.; Klotz, L.H.; Ramani, M.; Sugar, L.M.; Jacob, L.E.; Nam, R.K.; Fleshner, N.E. A Combination of Micronutrients Is Beneficial in Reducing the Incidence of Prostate Cancer and Increasing Survival in the Lady Transgenic Model. Cancer Prev. Res. 2009, 2, 473–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, J.; Jiang, C.; Deng, Y.; Özten, N.; Bosland, M.C. Cancer chemoprevention research with selenium in the post-SELECT era: Promises and challenges. Nutr. Cancer 2015, 68, 1–17. [Google Scholar] [CrossRef]
- Fritz, H.; Kennedy, D.; Fergusson, D.; Fernandes, R.; Cooley, K.; Seely, A.; Sagar, S.; Wong, R.; Seely, D. Selenium and Lung Cancer: A Systematic Review and Meta Analysis. PLoS ONE 2011, 6, e26259. [Google Scholar] [CrossRef] [Green Version]
- Kao, R.-H.; Lai, G.-M.; Chow, J.-M.; Liao, C.-H.; Zheng, Y.-M.; Tsai, W.-L.; Hsia, S.; Lai, I.-C.; Lee, H.-L.; Chuang, S.-E.; et al. Opposite Regulation of CHOP and GRP78 and Synergistic Apoptosis Induction by Selenium Yeast and Fish Oil via AMPK Activation in Lung Adenocarcinoma Cells. Nutrients 2018, 10, 1458. [Google Scholar] [CrossRef] [Green Version]
- Sugita, S.; Ito, K.; Yamashiro, Y.; Moriya, S.; Che, X.-F.; Yokoyama, T.; Hiramoto, M.; Miyazawa, K. EGFR-independent autophagy induction with gefitinib and enhancement of its cytotoxic effect by targeting autophagy with clarithromycin in non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2015, 461, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-C.; Kulp, S.K.; Wang, D.; Yang, C.-C.; Sargeant, A.M.; Hung, J.-H.; Kashida, Y.; Yamaguchi, M.; Chang, G.-D.; Chen, C.-S. Targeting Endoplasmic Reticulum Stress and Akt with OSU-03012 and Gefitinib or Erlotinib to Overcome Resistance to Epidermal Growth Factor Receptor Inhibitors. Cancer Res. 2008, 68, 2820–2830. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Gu, P.; Zhou, C.; Liang, A.; Ren, S.; Liu, F.; Zeng, Y.; Wu, Y.; Zhao, Y.; Huang, B.; et al. β-Catenin overexpression is associated with gefitinib resistance in non-small cell lung cancer cells. Pulm. Pharmacol. Ther. 2014, 28, 41–48. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, S.-Y.; Pyo, H. Cyclooxygenase-2 (COX-2) Negatively Regulates Expression of Epidermal Growth Factor Receptor and Causes Resistance to Gefitinib in COX-2-Overexpressing Cancer Cells. Mol. Cancer Res. 2009, 7, 1367–1377. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-H.; Tzeng, Y.-T.; Lai, G.-M.; Chang, C.-L.; Hu, M.-H.; Tsai, W.-L.; Liu, Y.-R.; Hsia, S.; Chuang, S.-E.; Chiou, T.-J.; et al. Omega-3 Fatty Acid-Enriched Fish Oil and Selenium Combination Modulates Endoplasmic Reticulum Stress Response Elements and Reverses Acquired Gefitinib Resistance in HCC827 Lung Adenocarcinoma Cells. Mar. Drugs 2020, 18, 399. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Wang, W.; Li, Y.; Ruden, U.M.; Wang, F.; Li, Y.; Wang, F.; Song, J.; Zheng, K. Low Molecular Weight Heparin Ablates Lung Cancer Cisplatin-Resistance by Inducing Proteasome-Mediated ABCG2 Protein Degradation. PLoS ONE 2012, 7, e41035. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.F.; Huang, Y.Y.; Wang, Y.J.; Gao, F.G. Upregulation of ABCG2 via the PI3K-Akt pathway contributes to acidic microenvironment-induced cisplatin resistance in A549 and LTEP-a-2 lung cancer cells. Oncol. Rep. 2016, 36, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, G.; Zhang, H.; Zhang, F.; Zhou, B.; Ning, F.; Wang, H.-S.; Cai, S.-H.; Du, J. Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/β-catenin/Snail signaling pathway. Eur. J. Pharmacol. 2014, 723, 156–166. [Google Scholar] [CrossRef]
- Kim, K.-C.; Lee, C. Reversal of Cisplatin Resistance by Epigallocatechin Gallate Is Mediated by Downregulation of Axl and Tyro 3 Expression in Human Lung Cancer Cells. Korean J. Physiol. Pharmacol. 2014, 18, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, K.-C.; Lee, C. Mistletoe (Viscum album) extract targets Axl to suppress cell proliferation and overcome cisplatin- and erlotinib-resistance in non-small cell lung cancer cells. Phytomedicine 2017, 36, 183–193. [Google Scholar] [CrossRef]
- Qi, W.; Chen, J.; Cheng, X.; Huang, J.; Xiang, T.; Li, Q.; Long, H.; Zhu, B. Targeting the Wnt-Regulatory Protein CTNNBIP1 by microRNA-214 Enhances the Stemness and Self-Renewal of Cancer Stem-Like Cells in Lung Adenocarcinomas. Stem Cells 2015, 33, 3423–3436. [Google Scholar] [CrossRef]
- Sun, F.-F.; Hu, Y.-H.; Xiong, L.-P.; Tu, X.-Y.; Zhao, J.-H.; Chen, S.-S.; Song, J.; Ye, X.-Q. Enhanced expression of stem cell markers and drug resistance in sphere-forming non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 6287–6300. [Google Scholar]
- Misuno, K.; Liu, X.; Feng, S.; Hu, S. Quantitative proteomic analysis of sphere-forming stem-like oral cancer cells. Stem Cell Res. Ther. 2013, 4, 156. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Shin, J.E.; Park, H.W. The Role of Hippo Pathway in Cancer Stem Cell Biology. Mol. Cells 2018, 41, 83–92. [Google Scholar] [CrossRef]
- Zimmermann, M.; Meyer, N. Annexin V/7-AAD Staining in Keratinocytes. Methods Mol. Biol. 2011, 740, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuthed, A.; Chantarawong, W.; Chanvorachote, P. Lung Cancer Stem Cells and Cancer Stem Cell-targeting Natural Compounds. Anticancer Res. 2018, 38, 3797–3809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eramo, A.; Lotti, F.; Sette, G.; Pilozzi, E.; Biffoni, M.; Di Virgilio, A.; Conticello, C.; Ruco, L.; Peschle, C.; De Maria, R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2007, 15, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer. Adv. Cancer Res. 2018, 137, 37–75. [Google Scholar] [CrossRef]
- Liu, X. The Epithelial-Mesenchymal Transition and Cancer Stem Cells: Functional and Mechanistic Links. Curr. Pharm. Des. 2015, 21, 1279–1291. [Google Scholar] [CrossRef]
- Byers, L.A.; Diao, L.; Wang, J.; Saintigny, P.; Girard, L.; Peyton, M.; Shen, L.; Fan, Y.; Giri, U.; Tumula, P.K.; et al. An Epithelial-Mesenchymal Transition Gene Signature Predicts Resistance to EGFR and PI3K Inhibitors and Identifies Axl as a Therapeutic Target for Overcoming EGFR Inhibitor Resistance. Clin. Cancer Res. 2013, 19, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-T.; Kim, Y.M.; Surh, Y.-J.; Baik, H.W.; Lee, S.-K.; Ha, J.; Park, O.J. Selenium Regulates Cyclooxygenase-2 and Extracellular Signal-Regulated Kinase Signaling Pathways by Activating AMP-Activated Protein Kinase in Colon Cancer Cells. Cancer Res. 2006, 66, 10057–10063. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Lee, Y.-K.; Kim, H.J.; Park, O.J.; Kim, Y.M. AMPK interacts with β-catenin in the regulation of hepatocellular carcinoma cell proliferation and survival with selenium treatment. Oncol. Rep. 2015, 35, 1566–1572. [Google Scholar] [CrossRef] [Green Version]
- Shigemi, Z.; Manabe, K.; Hara, N.; Baba, Y.; Hosokawa, K.; Kagawa, H.; Watanabe, T.; Fujimuro, M. Methylseleninic acid and sodium selenite induce severe ER stress and subsequent apoptosis through UPR activation in PEL cells. Chem. Interact. 2017, 266, 28–37. [Google Scholar] [CrossRef]
- Ke, B.; Wei, T.; Huang, Y.; Gong, Y.; Wu, G.; Liu, J.; Chen, X.; Shi, L. Interleukin-7 Resensitizes Non-Small-Cell Lung Cancer to Cisplatin via Inhibition of ABCG2. Mediat. Inflamm. 2019, 2019, 7241418. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, T.A.; Landreneau, R.J.; McKenna, R.J.; Santos, R.S.; Parker, R.J. Prevalence of In Vitro Extreme Chemotherapy Resistance in Resected Nonsmall-Cell Lung Cancer. Ann. Thorac. Surg. 2006, 81, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Wangpaichitr, M.; Wu, C.; Li, Y.Y.; Nguyen, D.J.M.; Kandemir, H.; Shah, S.; Chen, S.; Feun, L.G.; Prince, J.S.; Kuo, M.T.; et al. Exploiting ROS and metabolic differences to kill cisplatin resistant lung cancer. Oncotarget 2017, 8, 49275–49292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, W.; Shen, X.; Lei, J.; Xu, Q.; Yu, Y.; Li, R.; Wu, E.; Ma, Q. Hyperglycemia, a Neglected Factor during Cancer Progression. BioMed Res. Int. 2014, 2014, 461917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhang, X.; Sang, H.; Zhou, Y.; Shang, C.; Wang, Y.; Zhu, H. Effects of hyperglycemia on the progression of tumor diseases. J. Exp. Clin. Cancer Res. 2019, 38, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uprety, B.; Abrahamse, H. Targeting Breast Cancer and Their Stem Cell Population through AMPK Activation: Novel Insights. Cells 2022, 11, 576. [Google Scholar] [CrossRef]
- Wandee, J.; Prawan, A.; Senggunprai, L.; Kongpetch, S.; Tusskorn, O.; Kukongviriyapan, V. Metformin enhances cisplatin induced inhibition of cholangiocarcinoma cells via AMPK-mTOR pathway. Life Sci. 2018, 207, 172–183. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Tsichlis, P.N.; Struhl, K. Metformin Selectively Targets Cancer Stem Cells, and Acts Together with Chemotherapy to Block Tumor Growth and Prolong Remission. Cancer Res. 2009, 69, 7507–7511. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.R.; Chan, D.K.; Shank, J.J.; Griffith, K.A.; Fan, H.; Szulawski, R.; Yang, K.; Reynolds, R.K.; Johnston, C.; McLean, K.; et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight 2020, 5, e133247. [Google Scholar] [CrossRef]
- Saini, N.; Yang, X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim. Biophys. Sin. 2018, 50, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Cioce, M.; Pulito, C.; Strano, S.; Blandino, G.; Fazio, V.M. Metformin: Metabolic Rewiring Faces Tumor Heterogeneity. Cells 2020, 9, 2439. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.E.; Stratton, M.; Lillico, A.J.; Fakih, M.; Natarajan, R.; Clark, L.C.; Marshall, J.R. A report of high-dose selenium supplementation: Response and toxicities. J. Trace Elem. Med. Biol. 2004, 18, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kuriki, K.; Nagaya, T.; Tokudome, Y.; Imaeda, N.; Fujiwara, N.; Sato, J.; Goto, C.; Ikeda, M.; Maki, S.; Tajima, K.; et al. Plasma Concentrations of (n-3) Highly Unsaturated Fatty Acids Are Good Biomarkers of Relative Dietary Fatty Acid Intakes: A Cross-Sectional Study. J. Nutr. 2003, 133, 3643–3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, S.; Varghese, E.; Kubatka, P.; Triggle, C.; Büsselberg, D. Metformin: The Answer to Cancer in a Flower? Current Knowledge and Future Prospects of Metformin as an Anti-Cancer Agent in Breast Cancer. Biomolecules 2019, 9, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calza, G.; Nyberg, E.; Mäkinen, M.; Soliymani, R.; Cascone, A.; Lindholm, D.; Barborini, E.; Baumann, M.; Lalowski, M.; Eriksson, O. Lactate-Induced Glucose Output Is Unchanged by Metformin at a Therapeutic Concentration—A Mass Spectrometry Imaging Study of the Perfused Rat Liver. Front. Pharmacol. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajbaf, F.; De Broe, M.E.; Lalau, J.-D. Therapeutic Concentrations of Metformin: A Systematic Review. Clin. Pharmacokinet. 2015, 55, 439–459. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, S.; Zhang, W.-Q.; Yang, Y.-L.; Hang, P.; Wang, H.; Cheng, L.; Hsu, P.-C.; Wang, Y.-C.; Xu, Z.; et al. YAP1 regulates ABCG2 and cancer cell side population in human lung cancer cells. Oncotarget 2016, 8, 4096–4109. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Duan, W.; Liu, W.; Zhang, X.; Wang, Q. GRP78 in lung cancer. J. Transl. Med. 2021, 19, 118. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Dong, Y.; Park, Y.-M.; Ip, C. Endoplasmic Reticulum Stress Signal Mediators Are Targets of Selenium Action. Cancer Res. 2005, 65, 9073–9079. [Google Scholar] [CrossRef] [Green Version]
- Gopal, U.; Mowery, Y.; Young, K.; Pizzo, S.V. Targeting cell surface GRP78 enhances pancreatic cancer radiosensitivity through YAP/TAZ protein signaling. J. Biol. Chem. 2019, 294, 13939–13952. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Wu, T.-H.; Chiu, Y.-H.; Wang, H.; Li, T.-L.; Hsia, S.; Chan, Y.-L.; Wu, C.-J. Positive Effects of Preventive Nutrition Supplement on Anticancer Radiotherapy in Lung Cancer Bearing Mice. Cancers 2020, 12, 2445. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cui, J.; Wang, H.; Medina, R.; Zhang, S.; Zhang, X.; Zhuang, Z.; Lin, Y. Metformin enhances anti-cancer effects of cisplatin in meningioma through AMPK-mTOR signaling pathways. Mol. Ther. Oncolytics 2020, 20, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-Z.; Gao, Y.; Zhao, H.-W.; Zhou, M.; Chen, D.-L.; Tao, L.-T.; Guo, W.; Sun, L.-L.; Gu, C.-Y.; Chen, H.-R.; et al. Cordycepin Reverses Cisplatin Resistance in Non-small Cell Lung Cancer by Activating AMPK and Inhibiting AKT Signaling Pathway. Front. Cell Dev. Biol. 2021, 8, 609285. [Google Scholar] [CrossRef] [PubMed]
- Hampsch, R.A.; Wells, J.D.; Traphagen, N.A.; McCleery, C.F.; Fields, J.L.; Shee, K.; Dillon, L.M.; Pooler, D.B.; Lewis, L.D.; Demidenko, E.; et al. AMPK Activation by Metformin Promotes Survival of Dormant ER+ Breast Cancer Cells. Clin. Cancer Res. 2020, 26, 3707–3719. [Google Scholar] [CrossRef] [Green Version]
- Andugulapati, S.B.; Sundararaman, A.; Lahiry, M.; Rangarajan, A. AMP- activated protein kinase (AMPK) promotes breast cancer stemness and drug resistance. Dis. Models Mech. 2022, 15, dmm049203. [Google Scholar] [CrossRef]







| Cell-Cycle Distribution | |||||
|---|---|---|---|---|---|
| Treatment | Sub-G1 ( %) | G1 (%) | S (%) | G2/M (%) | |
| Parental A549 | Control | 1.64 | 74.01 | 10.28 | 13.94 |
| Parental A549 | Cisplatin 20 μg/mL | 24.77 | 61.54 | 7.71 | 5.69 |
| A549 sphere (in PluriSTEM™) | Control | 8.58 | 75.47 | 10.24 | 5.38 |
| A549 sphere (in PluriSTEM™) | Cisplatin 20 μg/mL | 10.1 | 80.44 | 7.29 | 2.13 |
| A549 sphere (in RPMI-1640) | Control | 6.13 | 81.23 | 6.09 | 6.46 |
| A549 sphere (in RPMI-1640) | Cisplatin 20 μg/mL | 8.19 | 76.96 | 10 | 4.66 |
| A549 Sphere Cells | Cell-Cycle Distribution | |||
|---|---|---|---|---|
| Treatment | Sub-G1 (%) | G1 (%) | S (%) | G2/M (%) |
| Control | 1.9 | 80.9 | 7.8 | 9.4 |
| Se-Y 200 ng/mL | 14.4 | 73.3 | 4.8 | 7.5 |
| FO 200 μM | 3.4 | 78.9 | 9.9 | 7.8 |
| Se-Y 200 ng/mL + FO 200 μM | 42.2 | 48.5 | 5.9 | 3.4 |
| Se-Y (ng/mL) | FO (μM) | FA (0–1) | CI |
|---|---|---|---|
| 50 | 50 | 0.368 | 0.389 |
| 50 | 200 | 0.334 | 1.078 |
| 200 | 50 | 0.412 | 0.941 |
| 200 | 200 | 0.727 | 0.645 |
| 400 | 50 | 0.624 | 0.968 |
| 400 | 200 | 0.835 | 0.656 |
| A549 Sphere | Cell-Cycle Distribution | |||
|---|---|---|---|---|
| Treatment | Sub-G1 (%) | G1 (%) | S (%) | G2/M (%) |
| Control | 9.37 | 67.53 | 15.32 | 6.43 |
| Se-Y 200 ng/mL + FO 200 μM | 45.87 | 44.15 | 6.44 | 2.43 |
| Cisplatin 20 μg/mL | 8.34 | 62.82 | 17.77 | 7.61 |
| Cisplatin 20 μg/mL + Se-Y 200 ng/mL + FO 200 μM | 63.02 | 24.53 | 5.58 | 2.67 |
| A549 Sphere | Cell-Cycle Distribution | |||
|---|---|---|---|---|
| Treatment | Sub-G1 (%) | G1 (%) | S (%) | G2/M (%) |
| Control | 8.46 | 73.9 | 6.84 | 10.28 |
| Se-Y 100 ng/mL + FO 100 μM | 37.77 | 47.48 | 7.66 | 6.09 |
| Cisplatin 20 μg/mL | 5.28 | 80.95 | 8.06 | 5.1 |
| Cisplatin 20 μg/mL + Se-Y 100 ng/mL + FO 100 μM | 71.58 | 22.13 | 4.51 | 0.82 |
| Cisplatin (μg/mL) | Se-Y (ng/mL) | FO (μM) | FA (0–1) | CI |
|---|---|---|---|---|
| 10 | 25 | 25 | 0.43 | 0.456 |
| 10 | 50 | 50 | 0.508 | 0.548 |
| 10 | 100 | 100 | 0.539 | 0.875 |
| 20 | 25 | 25 | 0.474 | 0.486 |
| 20 | 50 | 50 | 0.492 | 0.721 |
| 20 | 100 | 100 | 0.561 | 0.898 |
| 40 | 25 | 25 | 0.474 | 0.721 |
| 40 | 50 | 50 | 0.61 | 0.549 |
| 40 | 100 | 100 | 0.658 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, I.-C.; Liao, C.-H.; Hu, M.-H.; Chang, C.-L.; Lai, G.-M.; Chiou, T.-J.; Hsia, S.; Tsai, W.-L.; Lin, Y.-Y.; Chuang, S.-E.; et al. Selenium Yeast and Fish Oil Combination Diminishes Cancer Stem Cell Traits and Reverses Cisplatin Resistance in A549 Sphere Cells. Nutrients 2022, 14, 3232. https://doi.org/10.3390/nu14153232
Lai I-C, Liao C-H, Hu M-H, Chang C-L, Lai G-M, Chiou T-J, Hsia S, Tsai W-L, Lin Y-Y, Chuang S-E, et al. Selenium Yeast and Fish Oil Combination Diminishes Cancer Stem Cell Traits and Reverses Cisplatin Resistance in A549 Sphere Cells. Nutrients. 2022; 14(15):3232. https://doi.org/10.3390/nu14153232
Chicago/Turabian StyleLai, I-Chun, Chien-Huang Liao, Ming-Hung Hu, Chia-Lun Chang, Gi-Ming Lai, Tzeon-Jye Chiou, Simon Hsia, Wei-Lun Tsai, Yu-Yin Lin, Shuang-En Chuang, and et al. 2022. "Selenium Yeast and Fish Oil Combination Diminishes Cancer Stem Cell Traits and Reverses Cisplatin Resistance in A549 Sphere Cells" Nutrients 14, no. 15: 3232. https://doi.org/10.3390/nu14153232
APA StyleLai, I.-C., Liao, C.-H., Hu, M.-H., Chang, C.-L., Lai, G.-M., Chiou, T.-J., Hsia, S., Tsai, W.-L., Lin, Y.-Y., Chuang, S.-E., Whang-Peng, J., Chen, H.-Y., & Yao, C.-J. (2022). Selenium Yeast and Fish Oil Combination Diminishes Cancer Stem Cell Traits and Reverses Cisplatin Resistance in A549 Sphere Cells. Nutrients, 14(15), 3232. https://doi.org/10.3390/nu14153232