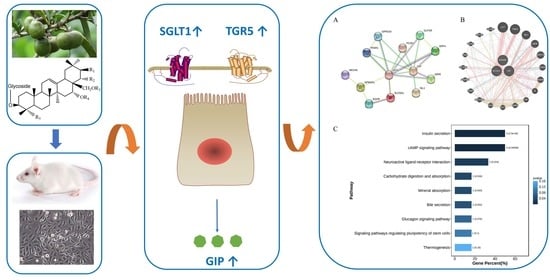
Saponins from Camellia sinensis Seeds Stimulate GIP Secretion in Mice and STC-1 Cells via SGLT1 and TGR5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Regents
2.2. Animal Experiment
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Ca2+ Measurement
2.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.7. Enzyme Linked Immunosorbent Assay (ELISA)
2.8. Western Blot Analysis
2.9. Transfection with Small Interfering RNA (siRNA)
2.10. Protein–Protein Interaction (PPI) Network Construction
2.11. Statistical Analysis
3. Results
3.1. Effect of TSS on GIP Expression in the Small Intestine of Mice
3.2. Effect of TSE1 on GIP Secretion in STC-1 Cells
3.3. Role of SGLT 1 in TSE1-Induced GIP Expression
3.4. Effect of TSE1 on TGR5 Expression in STC-1 Cells
3.5. Role of TGR5 in TSE1-Induced GIP Expression
3.6. PPI Network and KEGG Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaribeygi, H.; Maleki, M.; Butler, A.E.; Jamialahmadi, T.; Sahebkar, A. The impact of incretin-based medications on lipid metabolism. J. Diabetes. Res. 2021, 2021, 1815178. [Google Scholar] [CrossRef]
- Stemmer, K.; Finan, B.; DiMarchi, R.D.; Tschop, M.H.; Muller, T.D. Insights into incretin-based therapies for treatment of diabetic dyslipidemia. Adv. Drug Deliv. Rev. 2020, 159, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.M.; Akindehin, S.E.; Orsso, C.E.; Waldner, R.C.; DiMarchi, R.D.; Muller, T.D.; Haqq, A.M. Recent advances in incretin-based pharmacotherapies for the treatment of obesity and diabetes. Front. Endocrinol. 2022, 13, 838410. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.S.S.; Singh, S.K.; Rajendran, V.M.; Walk, S.T.; Coon, S.D. Regulation of glucose insulinotropic peptide and intestinal glucose transporters in the diet-induced obese mouse. J. Diabetes Res. 2022, 2022, 5636499. [Google Scholar] [CrossRef]
- Takagishi, M.; Aleogho, B.M.; Okumura, M.; Ushida, K.; Yamada, Y.; Seino, Y.; Fujimura, S.; Nakashima, K.; Shindo, A. Nutritional control of thyroid morphogenesis through gastrointestinal hormones. Curr. Biol. 2022, 32, 1485–1496.e4. [Google Scholar] [CrossRef] [PubMed]
- Mantelmacher, F.D.; Zvibel, I.; Cohen, K.; Epshtein, A.; Pasmanik-Chor, M.; Vogl, T.; Kuperman, Y.; Weiss, S.; Drucker, D.J.; Varol, C.; et al. GIP regulates inflammation and body weight by restraining myeloid-cell-derived S100A8/A9. Nat. Metab. 2019, 1, 58–69. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Holscher, C. GIP has neuroprotective effects in Alzheimer and Parkinson’s disease models. Peptides 2020, 125, 170184. [Google Scholar] [CrossRef]
- Ko, J.; Jang, S.; Kwon, W.; Kim, S.Y.; Jang, S.; Kim, E.; Ji, Y.R.; Park, S.; Kim, M.O.; Choi, S.K.; et al. Protective effect of GIP against monosodium glutamate-induced ferroptosis in mouse hippocampal HT-22 Cells through the MAPK signaling pathway. Antioxidants 2022, 11, 189. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Kumar, S.; Vijayan, M.; Bhatti, G.K.; Reddy, P.H. Therapeutic strategies for mitochondrial dysfunction and oxidative stress in age-related metabolic disorders. J. Diabetes Res. 2017, 146, 13–46. [Google Scholar]
- Pais, R.; Gribble, F.M.; Reimann, F. Stimulation of incretin secreting cells. Ther. Adv. Endocrinol. Metab. 2016, 7, 24–42. [Google Scholar] [CrossRef]
- Stepanov, V.; Stankov, K.; Mikov, M. The bile acid membrane receptor TGR5: A novel pharmacological target in metabolic, in- flammatory and neoplastic disorders. J. Recept. Signal Transduct. 2013, 33, 213–223. [Google Scholar] [CrossRef]
- Wang, L.Y.; Cheng, K.C.; Li, Y.; Niu, C.S.; Cheng, J.T.; Niu, H.S. Glycyrrhizic acid increases glucagon like peptide-1 secretion via TGR5 activation in type 1-like diabetic rats. Biomed. Pharmacother. 2017, 95, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, L.J.; Lenaerts, K.; Kiers, D.; Pais de Barros, J.P.; Le Guern, N.; Plesnik, J.; Thomas, C.; Bourgeois, T.; Dejong, C.H.C.; Kox, M.; et al. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep. 2017, 21, 1160–1168. [Google Scholar] [CrossRef]
- Guo, N.; Tong, T.T.; Ren, N.; Tu, Y.Y.; Li, B. Saponins from seeds of genus Camellia: Phytochemistry and bioactivity. Phytochemistry 2018, 149, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Jia, L.Y.; Wu, J.F.; Liu, Y.W.; Kang, H.; Liu, X.B.; Li, P.; He, P.M.; Tu, Y.Y.; Li, B. Simultaneous determination and quantification of triterpene saponins from Camellia sinensis seeds using UPLC-PDA-QTOF-MS/MS. Molecules 2019, 24, 3794. [Google Scholar] [CrossRef] [PubMed]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef]
- Hunt, J.E.; Billeschou, A.; Windelov, J.A.; Hartmann, B.; Ullmer, C.; Holst, J.J.; Kissow, H. Pharmacological activation of TGR5 promotes intestinal growth via a GLP-2-dependent pathway in mice. Am. J. Physiol.-Gastroint. Liver Physiol. 2020, 318, G980–G987. [Google Scholar] [CrossRef]
- Zhai, H.N.; Li, Z.; Peng, M.A.; Huang, Z.Q.; Qin, T.F.; Chen, L.X.; Li, H.B.; Zhang, H.; Zhang, W.Z.; Xu, G.Y. Takeda G protein-coupled receptor 5-mechanistic target of rapamycin Complex 1 signaling contributes to the increment of glucagon-like peptide-1 production after Roux-en-Y gastric bypass. Ebiomedicine 2018, 32, 201–214. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Ussher, J.R.; Greenwell, A.A.; Nguyen, M.A.; Mulvihill, E.E. Cardiovascular effects of incretin-based therapies: Integrating Mechanisms with cardiovascular outcome trials. Diabetes 2022, 71, 173–183. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Sathyapalan, T.; Banach, M.; Sahebkar, A. Incretins and microRNAs: Interactions and physiological relevance. Pharmacol. Res. 2020, 153, 104662. [Google Scholar] [CrossRef] [PubMed]
- Hajishafiee, M.; McVeay, C.; Lange, K.; Rehfeld, J.F.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal infusion of lauric acid and L-tryptophan, alone and combined, on glucoregulatory hormones, gastric emptying and glycaemia in healthy men. Metab.-Clin. Exp. 2022, 129, 155140. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, R.; Raza, G.S.; Makila, E.; Riikonen, J.; Kovalainen, M.; Ueta, Y.; Lehto, V.P.; Salonen, J.; Herzig, K.H. Colonic delivery of alpha-linolenic acid by an advanced nutrient delivery system prolongs glucagon-like peptide-1 secretion and inhibits food intake in Mice. Mol. Nutr. Food Res. 2022, 66, e2100978. [Google Scholar] [CrossRef] [PubMed]
- Martchenko, A.; Biancolin, A.D.; Martchenko, S.E.; Brubaker, P.L. Nobiletin ameliorates high fat-induced disruptions in rhythmic glucagon-like peptide-1 secretion. Sci. Rep. 2022, 12, 7271. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Hu, M.Y.; Guo, H.F.; Li, J.; Yu, Y.L.; Jin, S.; Wang, X.T.; Liu, L.; Liu, X.D. Increased glucagon-like peptide-1 secretion may be involved in antidiabetic effects of ginsenosides. J. Endocrinol. 2013, 217, 185–196. [Google Scholar] [CrossRef]
- Song, P.; Onishi, A.; Koepsell, H.; Vallon, V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin. Ther. Targets 2016, 20, 1109–1125. [Google Scholar] [CrossRef]
- Gorboulev, V.; Schurmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose-absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Coon, S.D.; Rajendran, V.M.; Singh, S.K. Prolonged high-fat feeding decreases SGLT1 and GLUT2 activity and blunts their upregulation by glucose-dependent insulinotropic polypeptide (GIP) in obese mice. Gastroenterology 2014, 146, S493. [Google Scholar] [CrossRef]
- Ogata, H.; Seino, Y.; Harada, N.; Iida, A.; Suzuki, K.; Izumoto, T.; Ishikawa, K.; Uenishi, E.; Ozaki, N.; Hayashi, Y.; et al. K-ATP channel as well as SGLT1 participates in GIP secretion in the diabetic state. J. Endocrinol. 2014, 222, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Mace, O.J.; Schindler, M.; Patel, S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 2012, 590, 2917–2936. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, F.; Song, X.D.; Shi, M.; Liang, G.D.; Zhang, L.R.; Huang, F.; Jiang, G.R. 3-Deoxyglucosone reduces glucagon-like peptide-1 secretion at low glucose levels through down-regulation of SGLT1 expression in STC-1 cells. Arch. Physiol. Biochem. 2021, 127, 311–317. [Google Scholar] [CrossRef]
- Cipriani, S.; Mencarelli, A.; Chini, M.G.; Distrutti, E.; Renga, B.; Bifulco, G.; Baldelli, F.; Donini, A.; Fiorucci, S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS ONE 2011, 6, e25637. [Google Scholar] [CrossRef]
- Chavez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef] [PubMed]
- Merlen, G.; Bidault-Jourdainne, V.; Kahale, N.; Glenisson, M.; Ursic-Bedoya, J.; Doignon, I.; Garcin, I.; Humbert, L.; Rainteau, D.; Tordjmann, T. Hepatoprotective impact of the bile acid receptor TGR5. Liver Int. 2020, 40, 1005–1015. [Google Scholar] [CrossRef]
- Garibay, D.; Zaborska, K.E.; Shanahan, M.; Zheng, Q.N.; Kelly, K.M.; Montrose, D.C.; Dannenberg, A.J.; Miller, A.D.; Sethupathy, P.; Cummings, B.P. TGR5 protects against colitis in mice, but vertical sleeve gastrectomy increases colitis severity. Obes. Surg. 2019, 29, 1593–1601. [Google Scholar] [CrossRef]
- Maczewsky, J.; Kaiser, J.; Gresch, A.; Gerst, F.; Dufer, M.; Krippeit-Drews, P.; Drews, G. TGR5 activation promotes stimulus-secretion coupling of pancreatic -cells via a PKA-dependent pathway. Diabetes 2019, 68, 324–336. [Google Scholar] [CrossRef]
- Lo, S.H.; Cheng, K.C.; Li, Y.X.; Chang, C.H.; Cheng, J.T.; Lee, K.S. Development of betulinic acid as an agonist of TGR5 receptor using a new in vitro assay. Drug Des. Dev. Ther. 2016, 10, 2669–2676. [Google Scholar]
- Athanasios, A.; Charalampos, V.; Vasileios, T.; Ashraf, G.M. Protein-Protein interaction (PPI) network: Recent advances in drug discovery. Curr. Drug Metab. 2017, 18, 5–10. [Google Scholar] [CrossRef]
- Guo, T.; Bai, Y.H.; Cheng, X.J.; Han, H.B.; Du, H.; Hu, Y.; Jia, S.Q.; Xing, X.F.; Ji, J.F. Insulin gene enhancer protein 1 mediates glycolysis and tumorigenesis of gastric cancer through regulating glucose transporter 4. Cancer Commun. 2021, 41, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Mayendraraj, A.; Rosenkilde, M.M.; Gasbjerg, L.S. GLP-1 and GIP receptor signaling in beta cells—A review of receptor interactions and co-stimulation. Peptides 2022, 151, 170749. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Larraufie, P.F.; Goldspink, D.A.; Gribble, F.M.; Reimann, F. Chylomicrons stimulate incretin secretion in mouse and human cells. Diabetologia 2017, 60, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Cvijanovic, N.; Isaacs, N.J.; Rayner, C.K.; Feinle-Bisset, C.; Young, R.L.; Little, T.J. Lipid stimulation of fatty acid sensors in the human duodenum: Relationship with gastrointestinal hormones, BMI and diet. Int. J. Obes. 2017, 41, 233–239. [Google Scholar] [CrossRef] [PubMed]
- El, K.; Campbell, J.E. The role of GIP in alpha-cells and glucagon secretion. Peptides 2020, 125, 170213. [Google Scholar] [CrossRef]
- Aerts, L.; Terry, N.A.; Sainath, N.N.; Torres, C.; Martin, M.G.; Ramos-Molina, B.; Creemers, J.W. Novel homozygous inactivating mutation in the PCSK1 gene in an infant with congenital malabsorptive diarrhea. Genes 2021, 12, 710. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Huang, S.F.; Li, P.P.; Chen, Q.; Li, Y.Z.; Zhou, Y.Z.; Wang, L.T.; Kang, M.X.; Zhang, B.; Yang, B.; et al. Pancreatic cancer-derived exosomes suppress the production of GIP and GLP-1 from STC-1 cells in vitro by down-regulating the PCSK1/3. Cancer Lett. 2018, 431, 190–200. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Wang, K.; Chen, S.; Kang, J.; Guo, N.; Chen, H.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; et al. Saponins from Camellia sinensis Seeds Stimulate GIP Secretion in Mice and STC-1 Cells via SGLT1 and TGR5. Nutrients 2022, 14, 3413. https://doi.org/10.3390/nu14163413
Zhu H, Wang K, Chen S, Kang J, Guo N, Chen H, Liu J, Wu Y, He P, Tu Y, et al. Saponins from Camellia sinensis Seeds Stimulate GIP Secretion in Mice and STC-1 Cells via SGLT1 and TGR5. Nutrients. 2022; 14(16):3413. https://doi.org/10.3390/nu14163413
Chicago/Turabian StyleZhu, Huanqing, Kaixi Wang, Shuna Chen, Jiaxin Kang, Na Guo, Hongbo Chen, Junsheng Liu, Yuanyuan Wu, Puming He, Youying Tu, and et al. 2022. "Saponins from Camellia sinensis Seeds Stimulate GIP Secretion in Mice and STC-1 Cells via SGLT1 and TGR5" Nutrients 14, no. 16: 3413. https://doi.org/10.3390/nu14163413
APA StyleZhu, H., Wang, K., Chen, S., Kang, J., Guo, N., Chen, H., Liu, J., Wu, Y., He, P., Tu, Y., & Li, B. (2022). Saponins from Camellia sinensis Seeds Stimulate GIP Secretion in Mice and STC-1 Cells via SGLT1 and TGR5. Nutrients, 14(16), 3413. https://doi.org/10.3390/nu14163413