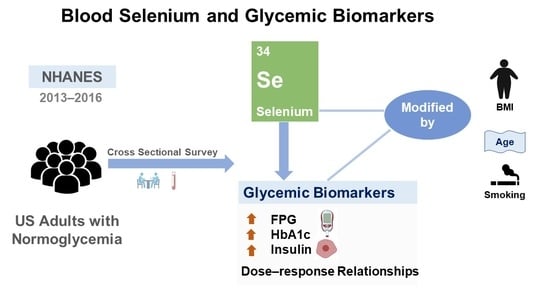
Cross-Sectional Association of Blood Selenium with Glycemic Biomarkers among U.S. Adults with Normoglycemia in the National Health and Nutrition Examination Survey 2013–2016
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements of Se and Other Metals
2.3. Measurements of FPG, HbA1c, OGTT, and Insulin
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Overall Associations of Blood Se and Glycemic Biomarkers
3.3. Subgroup Analyses
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, A.C.; Caballero, B.; Cousins, R.J.; Tucker, K.L.; Ziegler, T.R. Modern Nutrition in Health and Disease, 7th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2012. [Google Scholar]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Hauffe, R.; Stein, V.; Chudoba, C.; Flore, T.; Rath, M.; Ritter, K.; Schell, M.; Wardelmann, K.; Deubel, S.; Kopp, J.F.; et al. GPx3 dysregulation impacts adipose tissue insulin receptor expression and sensitivity. JCI Insight 2020, 5, e136283. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Wise, L.A.; Rothman, K.J. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021, 197, 111210. [Google Scholar] [CrossRef]
- Stranges, S.; Marshall, J.R.; Navas-Acien, A.; Guallar, E.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes A: A randomized trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef]
- Moon, S.; Chung, H.S.; Yu, J.M.; Yoo, H.J.; Park, J.H.; Kim, D.S.; Park, Y.-K.; Yoon, S.N. Association between serum selenium level and the prevalence of diabetes mellitus in U.S. population. J. Trace Elem. Med. Biol. 2019, 52, 83–88. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, C.; Yang, Z.; Zhang, W.; Niu, Y.; Li, X.; Qin, L.; Su, Q. Alterations of serum trace elements in patients with type 2 diabetes. J. Trace Elem. Med. Biol. 2017, 40, 91–96. [Google Scholar] [CrossRef]
- Gupta, M.K. Glycemic biomarkers as tools for diagnosis and monitoring of diabetes. MLO Med. Lab. Obs. 2013, 45, 8–14. [Google Scholar]
- National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2016. Available online: https://wwwn.cdc.gov/Nchs/data/nhanes/analyticguidelines/11-16-analytic-guidelines.pdf (accessed on 13 September 2022).
- Cadmium, Lead, Manganese, Mercury, and Selenium Lab Procedure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/PBCD_I_met.pdf (accessed on 13 September 2022).
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Yang, J.; Chan, K.; Choi, C.; Yang, A.; Lo, K. Identifying Effects of urinary metals on type 2 diabetes in U.S. adults: Cross-sectional analysis of National Health and Nutrition Examination Survey 2011–2016. Nutrients 2022, 14, 1552. [Google Scholar] [CrossRef]
- Lo, K.; Yang, J.L.; Chen, C.L.; Liu, L.; Huang, Y.Q.; Feng, Y.Q.; Yang, A.M. Associations between blood and urinary manganese with metabolic syndrome and its components: Cross-sectional analysis of National Health and Nutrition Examination Survey 2011–2016. Sci. Total Environ. 2021, 780, 146527. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, A.; Cheng, N.; Huang, W.; Huang, P.; Liu, N.; Bai, Y. Sex-specific associations of blood and urinary manganese levels with glucose levels, insulin resistance and kidney function in US adults: National health and nutrition examination survey 2011-2016. Chemosphere 2020, 258, 126940. [Google Scholar] [CrossRef]
- Kim, J.; Chung, H.S.; Choi, M.K.; Roh, Y.K.; Yoo, H.J.; Park, J.H.; Kim, D.S.; Yu, J.M.; Moon, S. Association between serum selenium level and the presence of diabetes mellitus: A meta-analysis of observational studies. Diabetes Metab. J. 2019, 43, 447–460. [Google Scholar] [CrossRef]
- Ogawa-Wong, A.N.; Berry, M.J.; Seale, L.A. Selenium and metabolic disorders: An emphasis on type 2 diabetes risk. Nutrients 2016, 8, 80. [Google Scholar] [CrossRef]
- Lin, J.; Shen, T. Association of dietary and serum selenium concentrations with glucose level and risk of diabetes mellitus: A cross sectional study of national health and nutrition examination survey, 1999–2006. J. Trace Elem. Med. Biol. 2021, 63, 126660. [Google Scholar] [CrossRef] [PubMed]
- Hrafnkelsson, H.; Magnusson, K.T.H.; Sigurdsson, E.L.; Johannsson, E. Association of BMI and fasting insulin with cardiovascular disease risk factors in seven-year-old Icelandic children. Scand. J. Prim. Health Care 2009, 27, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Osuna, J.A.; Gómez-Pérez, R.; Arata-Bellabarba, G.; Villaroel, V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch. Androl. 2006, 52, 355–361. [Google Scholar] [CrossRef]
- Campbell, S.C.; Aldibbiat, A.; Marriott, C.E.; Landy, C.; Ali, T.; Ferris, W.F.; Butler, C.S.; Shaw, J.A.; Macfarlane, W.M. Selenium stimulates pancreatic beta-cell gene expression and enhances islet function. FEBS Lett. 2008, 582, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Rocourt, C.R.B.; Cheng, W.-H. Selenium supranutrition: Are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance? Nutrients 2013, 5, 1349–1365. [Google Scholar] [CrossRef]
- Song, X. The Impact of Heat Shock Protein 72 Expression on Mitochondrial Function and Insulin Action. Master’s Thesis, University of California, Los Angeles, CA, USA, 2014. [Google Scholar]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Campaner, A.B.; Nadais, R.F.; Galvão, M.A.L. The effect of cigarette smoking on cervical langerhans cells and T and B lymphocytes in normal uterine cervix epithelium. Int. J. Gynecol. Pathol. 2009, 28, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.A.; Xiang, A.H.; Azen, S.P.; Peters, R.K.; Kjos, S.L.; Marroquin, A.; Goico, J.; Ochoa, C.; Tan, S.; Berkowitz, K.; et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002, 51, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Gromadzinska, J.; Sobala, W.; Reszka, E.; Wasowicz, W. Lung cancer risk associated with selenium status is modified in smoking individuals by Sep15 polymorphism. Eur. J. Nutr. 2008, 47, 47–54. [Google Scholar] [CrossRef] [PubMed]



| Variables a | Overall (n = 2706) | Men (n = 1396) | Women (n = 1310) | p Value |
|---|---|---|---|---|
| Age Group, years | 0.154 | |||
| 18–39 | 1185 (43.8) | 635 (45.5) | 550 (42.0) | |
| 40–59 | 923 (34.1) | 456 (32.7) | 467 (35.6) | |
| ≥60 | 598 (22.1) | 305 (21.8) | 293 (22.4) | |
| Race/Hispanic origin | 0.064 | |||
| Non-Hispanic White | 1177 (43.5) | 578 (41.4) | 599 (45.7) | |
| Non-Hispanic Black | 524 (19.4) | 287 (20.6) | 237 (18.1) | |
| Other Hispanic | 656 (24.2) | 337 (24.1) | 319 (24.4) | |
| Other Race | 349 (12.9) | 194 (13.9) | 155 (11.8) | |
| Education level | <0.001 | |||
| Less than high school | 437 (16.1) | 266 (19.1) | 171 (13.1) | |
| High school | 592 (21.9) | 335 (24.0) | 257 (19.6) | |
| At least some college | 1677 (62.0) | 795 (56.9) | 882 (67.3) | |
| Poverty to income ratio | 0.507 | |||
| <1 | 640 (23.7) | 338 (24.2) | 302 (23.1) | |
| ≥1 | 2066 (76.3) | 1058 (75.8) | 1008 (76.9) | |
| Smoking status | <0.001 | |||
| Never smoker | 1468 (54.2) | 655 (46.9) | 813 (62.1) | |
| Former smoker | 622 (23.0) | 378 (27.1) | 244 (18.6) | |
| Current smoker | 616 (22.8) | 363 (26.0) | 253 (19.3) | |
| Alcohol drinking | <0.001 | |||
| 2 drinks or less per day | 1850 (63.0) | 721 (51.6) | 984 (75.1) | |
| 3 drinks or above per day | 1001 (37.0) | 675 (48.4) | 326 (24.9) | |
| Body mass index in kg/m2 | <0.001 | |||
| ≤25 | 860 (31.8) | 414 (29.7) | 446 (34.0) | |
| 25.1–29.9 | 900 (33.3) | 546 (39.1) | 354 (27.0) | |
| ≥30 | 946 (35.0) | 436 (31.2) | 510 (38.9) | |
| Average daily energy intake, kcal | <0.001 | |||
| Q1 (<1440) | 651 (24.1) | 214 (15.3) | 437 (33.4) | |
| Q2 (1440–1950) | 617 (22.8) | 280 (20.1) | 337 (25.7) | |
| Q3 (1950–2590) | 696 (25.7) | 372 (26.6) | 324 (24.7) | |
| Q4 (≥2590) | 742 (27.4) | 530 (38.0) | 212 (16.2) | |
| Hypertension history | 0.353 | |||
| No | 1789 (66.1) | 911 (65.3) | 878 (67.0) | |
| Yes | 917 (33.9) | 485 (34.7) | 432 (33.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Chen, E.; Choi, C.; Chan, K.; Yang, Q.; Rana, J.; Yang, B.; Huang, C.; Yang, A.; Lo, K. Cross-Sectional Association of Blood Selenium with Glycemic Biomarkers among U.S. Adults with Normoglycemia in the National Health and Nutrition Examination Survey 2013–2016. Nutrients 2022, 14, 3972. https://doi.org/10.3390/nu14193972
Yang J, Chen E, Choi C, Chan K, Yang Q, Rana J, Yang B, Huang C, Yang A, Lo K. Cross-Sectional Association of Blood Selenium with Glycemic Biomarkers among U.S. Adults with Normoglycemia in the National Health and Nutrition Examination Survey 2013–2016. Nutrients. 2022; 14(19):3972. https://doi.org/10.3390/nu14193972
Chicago/Turabian StyleYang, Jingli, En Chen, Cheukling Choi, Kayue Chan, Qinghua Yang, Juwel Rana, Bo Yang, Chuiguo Huang, Aimin Yang, and Kenneth Lo. 2022. "Cross-Sectional Association of Blood Selenium with Glycemic Biomarkers among U.S. Adults with Normoglycemia in the National Health and Nutrition Examination Survey 2013–2016" Nutrients 14, no. 19: 3972. https://doi.org/10.3390/nu14193972
APA StyleYang, J., Chen, E., Choi, C., Chan, K., Yang, Q., Rana, J., Yang, B., Huang, C., Yang, A., & Lo, K. (2022). Cross-Sectional Association of Blood Selenium with Glycemic Biomarkers among U.S. Adults with Normoglycemia in the National Health and Nutrition Examination Survey 2013–2016. Nutrients, 14(19), 3972. https://doi.org/10.3390/nu14193972