Improved Endurance Running Performance Following Haskap Berry (Lonicera caerulea L.) Ingestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
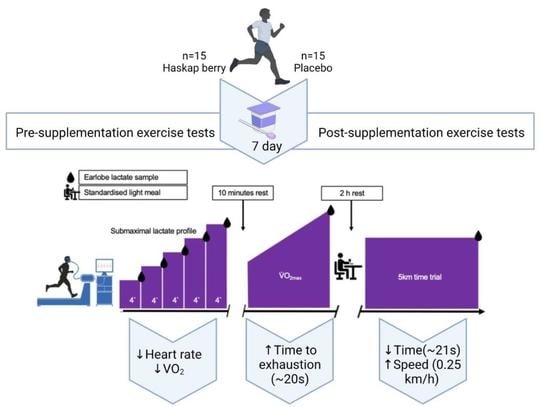
2.2. Study Design
2.3. Experimental Trials
2.4. Measurements
2.5. Treatment and Dietary Control
2.6. Power Calculation and Statistical Analysis
3. Results
Submaximal Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, D. Medical Herbalism: The Science and Practice of Herbal Medicine; Simon and Schuster: New York, NY, USA, 2003. [Google Scholar]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Moffat, A.C. History of doping in sport. In Sport and Exercise Medicine for Pharmacists; Pharmaceutical Press: London, UK, 2006; pp. 219–237. [Google Scholar]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef] [Green Version]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Bajes, H.R.; Al-Dujaili, E.A. Polyphenolic-rich Fruits and Supplements Enhance Exercise Performance; General Review. Jordan J. Pharm. Sci. 2017, 10, 135–151. [Google Scholar]
- Cook, M.D.; Willems, M.E.T. Dietary Anthocyanins: A Review of the Exercise Performance Effects and Related Physiological Responses. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Bell, P.G.; McHugh, M.P.; Stevenson, E.; Howatson, G. The role of cherries in exercise and health. Scand. J. Med. Sci. Sports 2014, 24, 477–490. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 802. [Google Scholar] [CrossRef] [Green Version]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Istas, G.; Boschek, L.; Feliciano, R.P.; Mills, C.E.; Boby, C.; Gómez-Alonso, S.; Milenkovic, D.; Heiss, C. Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights from Randomized Controlled Trials, Metabolomics, and Nutrigenomics. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2019, 74, 967–976. [Google Scholar] [CrossRef]
- Cook, M.; Myers, S.D.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.T.; Barton, M.J.; Bowtell, J.L. Montmorency cherry supplementation improves 15-km cycling time-trial performance. Eur. J. Appl. Physiol. 2019, 119, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency Cherries Reduce the Oxidative Stress and Inflammatory Responses to Repeated Days High-Intensity Stochastic Cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Arumuggam, N.; Amararathna, M.; De Silva, A. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Gołba, M.; Sokół-Łętowska, A.; Kucharska, A.Z. Health Properties and Composition of Honeysuckle Berry Lonicera caerulea L. An Update on Recent Studies. Molecules 2020, 25, 749. [Google Scholar] [CrossRef] [Green Version]
- Minami, M.; Nakamura, M. Effect of Haskap (Lonicera caerulea) on streptococcus pneumoniae infected aged-mouse. Int. J. Infect. Dis. 2020, 101, 4–5. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [Green Version]
- Edwards, M.; Czank, C.; Woodward, G.M.; Cassidy, A.; Kay, C.D. Phenolic Metabolites of Anthocyanins Modulate Mechanisms of Endothelial Function. J. Agric. Food Chem. 2015, 63, 2423–2431. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-W.; Ikeda, K.; Yamori, Y. Cyanidin-3-glucoside regulates phosphorylation of endothelial nitric oxide synthase. FEBS Lett. 2004, 574, 176–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, L.; Williams, C.M. A pilot dose–response study of the acute effects of haskap berry extract (Lonicera caerulea L.) on cognition, mood, and blood pressure in older adults. Eur. J. Nutr. 2019, 58, 3325–3334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsukawa, T.; Motojima, H.; Sato, Y.; Takahashi, S.; Villareal, M.O.; Isoda, H. Upregulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance. Sci. Rep. 2017, 7, 44799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saclier, M.; Bonfanti, C.; Antonini, S.; Angelini, G.; Mura, G.; Zanaglio, F.; Taglietti, V.; Romanello, V.; Sandri, M.; Tonelli, C.; et al. Nutritional intervention with cyanidin hinders the progression of muscular dystrophy. Cell Death Dis. 2020, 11, 127. [Google Scholar] [CrossRef] [Green Version]
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol. 2008, 586, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Quirk, H.; Bullas, A.; Haake, S.; Goyder, E.; Graney, M.; Wellington, C.; Copeland, R.; Reece, L.; Stevinson, C. Exploring the benefits of participation in community-based running and walking events: A cross-sectional survey of parkrun participants. BMC Public Health 2021, 21, 1978. [Google Scholar] [CrossRef]
- Reinders, A.; Reggiori, F.; Shennan, A.H. Validation of the DINAMAP ProCare blood pressure device according to the international protocol in an adult population. Blood Press. Monit. 2006, 11, 293–296. [Google Scholar] [CrossRef]
- O’Brien, E.; Asmar, R.; Beilin, L.; Imai, Y.; Mallion, J.-M.; Mancia, G.; Mengden, T.; Myers, M.; Padfield, P.; Palatini, P.; et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J. Hypertens. 2003, 21, 821–848. [Google Scholar] [CrossRef]
- Jones, A.M.; Doust, J.H. A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J. Sports Sci. 1996, 14, 321–327. [Google Scholar] [CrossRef]
- Smith, C.G.; Jones, A.M. The relationship between critical velocity, maximal lactate steady-state velocity and lactate turnpoint velocity in runners. Eur. J. Appl. Physiol. 2001, 85, 19–26. [Google Scholar] [CrossRef]
- Jones, A.M.; Winter, E.M.; Davison, R.R.; Bromley, P.D.; Mercer, T. Sport and Exercise Physiology Testing Guidelines: The British Association of Sport and Exercise Sciences Guide; Routledge: London, UK, 2016. [Google Scholar]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- Oksuz, T.; Tacer-Caba, Z.; Nilufer-Erdil, D.; Boyacioglu, D. Changes in bioavailability of sour cherry (Prunus cerasus L.) phenolics and anthocyanins when consumed with dairy food matrices. J. Food Sci. Technol. 2019, 56, 4177–4188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2016; 55, 1695–1705. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Ishisaka, A.; Mawatari, K.; Vidal-Diez, A.; Spencer, J.P.E.; Terao, J. Blueberry intervention improves vascular reactivity and lowers blood pressure in high-fat-, high-cholesterol-fed rats. Br. J. Nutr. 2013, 109, 1746–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braakhuis, A.J.; Somerville, V.X.; Hurst, R.D. The effect of New Zealand blackcurrant on sport performance and related biomarkers: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Adriouch, S.; Lampuré, A.; Nechba, A.; Baudry, J.; Assmann, K.; Kesse-Guyot, E.; Hercberg, S.; Scalbert, A.; Touvier, M.; Fezeu, L.K. Prospective Association between Total and Specific Dietary Polyphenol Intakes and Cardiovascular Disease Risk in the Nutrinet-Santé French Cohort. Nutrients 2018, 10, 1587. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.; Clark, T.; Newman-Judd, K.; Arnold, J.; Steele, J. Intra-Subject Variability of 5 km Time Trial Performance Completed by Competitive Trained Runners. J. Hum. Kinet. 2017, 57, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Hillsdale, N.J., Ed.; L. Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Kimble, R.; Jones, K.; Howatson, G. The effect of dietary anthocyanins on biochemical, physiological, and subjective exercise recovery: A systematic review and meta-analysis. Crit. Rev. Food. Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Somerville, V.; Bringans, C.; Braakhuis, A. Polyphenols and Performance: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 1589–1599. [Google Scholar] [CrossRef]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute Dietary Nitrate Supplementation Improves Cycling Time Trial Performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.A.; Cook, M.D.; Willems, M.E.T. Effect of New Zealand Blackcurrant Extract on Repeated Cycling Time Trial Performance. Sports 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Perkins, I.C.; Vine, S.A.; Blacker, S.D.; Willems, M. New Zealand Blackcurrant Extract Improves High-Intensity Intermittent Running. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.E.; Cousins, L.; Williams, D.; Blacker, S.D. Beneficial Effects of New Zealand Blackcurrant Extract on Maximal Sprint Speed during the Loughborough Intermittent Shuttle Test. Sports 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, K.M.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M.; Howatson, G. Effects of Montmorency Tart Cherry (L. Prunus Cerasus) Consumption on Nitric Oxide Biomarkers and Exercise Performance. Scand J. Med. Sci. Sports Exerc. 2018, 50, 720. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Antioxidant supplementation, redox deficiencies and exercise performance: A falsification design. Free Radic. Biol. Med. 2020, 158, 44–52. [Google Scholar] [CrossRef]
- Reid, M.B. Redox interventions to increase exercise performance. J. Physiol. 2015, 594, 5125–5133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehfus, L.R.; Gillespie, Z.E.; Almendáriz-Palacios, C.; Low, N.H.; Eskiw, C.H. Haskap Berry Phenolic Subclasses Differentially Impact Cellular Stress Sensing in Primary and Immortalized Dermal Fibroblasts. Cells 2021, 10, 2643. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-K.; Surh, Y.-J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [Green Version]
- Wangdi, J.T.; O’Leary, M.F.; Kelly, V.G.; Jackman, S.R.; Tang, J.C.Y.; Dutton, J.; Bowtell, J.L. Tart Cherry Supplement Enhances Skeletal Muscle Glutathione Peroxidase Expression and Functional Recovery after Muscle Damage. Med. Sci. Sports Exerc. 2021. [Google Scholar] [CrossRef] [PubMed]



| Age (years) | Stature (cm) | Mass (kg) | 5 km TT (s) | Training Volume (min/week) | |
|---|---|---|---|---|---|
| Haskap (n = 15) | 30 ± 8 | 176.5 ± 5.3 | 75.0 ± 10.9 | 1377 ± 192 | 281 ± 142 |
| Control (n =15) | 35 ± 6 | 179.8 ± 8.6 | 80.4 ± 10.0 | 1299 ± 141 | 245 ± 156 |
| ANCOVA Adjusted for Baseline | ||||||
|---|---|---|---|---|---|---|
| Control | Haskap | Difference (95% CI) | F | p-Value | Effect Size η2 | |
| Speed @LT (km/h) | ||||||
| Pre | 11.7 ±1.9 | 12.1 ± 1.8 | 0.26 (−0.68–0.22) | 1.062 | 0.312 | 0.038 |
| Post | 11.7 ± 1.9 | 12.4 ± 1.7 | ||||
| HR @LT (bpm) * | ||||||
| Pre | 158 ± 10 | 151 ± 10 | 3.3 (0.67–5.98) | 6.639 | 0.016 | 0.197 |
| Post | 159 ± 10 | 149 ± 9 | ||||
| RPE @LT | ||||||
| Pre | 11.6 ± 2.1 | 12.1 ± 2.3 | 0.03 (−0.94–0.88) | 0.005 | 0.944 | <0.001 |
| Post | 11.4 ± 2.1 | 11.8 ± 2.0 | ||||
| Relative O2 @LT (mL/kg/min) * | ||||||
| Pre | 39.6 ± 5.7 | 40.7 ± 5.0 | 2.2 (0.67–3.69) | 8.799 | 0.006 | 0.246 |
| Post | 40.4 ± 5.6 | 39.3 ± 5.3 | ||||
| Absolute O2 @LT (mL) * | ||||||
| Pre | 2946 ± 468 | 3248 ± 413 | 131 (16.6–246) | 5.517 | 0.026 | 0.170 |
| Post | 2998 ± 404 | 3139 ± 440 | ||||
| ANCOVA Adjusted for Baseline | ||||||
|---|---|---|---|---|---|---|
| Control | Haskap | Difference (95% CI) | F | p-Value | Effect Size η2 | |
| Speed @LTP (km/h) | ||||||
| Pre | 13.3 ± 1.7 | 14.1 ± 1.6 | 0.177 (−0.20–0.56) | 0.925 | 0.345 | 0.033 |
| Post | 13.5 ±1.7 | 14.0 ± 1.7 | ||||
| HR @LTP (bpm) * | ||||||
| Pre | 171 ± 8 | 169 ± 7 | 5.3 (2.82–7.69) | 19.534 | <0.001 | 0.420 |
| Post | 172 ± 8 | 165 ± 6 | ||||
| RPE @LTP | ||||||
| Pre | 14.9 ± 1.5 | 14.7 ± 2.2 | 0.14 (−0.49–0.76) | 0.201 | 0.657 | 0.007 |
| Post | 15.0 ± 1.2 | 14.7 ± 2.1 | ||||
| Relative O2 @LTP (mL/kg/min) | ||||||
| Pre | 44.6 ± 6.1 | 46.0 ± 5.0 | 0.6 (−0.84–2.11) | 0.786 | 0.383 | 0.028 |
| Post | 45.2 ± 6.3 | 45.8 ± 5.1 | ||||
| Absolute O2 @LTP (mL) | ||||||
| Pre | 3328 ± 522 | 3676 ± 403 | 0.45 (−112.10–113.01) | <0.001 | 0.993 | <0.001 |
| Post | 3358 ± 478 | 3656 ± 378 | ||||
| ANCOVA Adjusted for Baseline | ||||||
|---|---|---|---|---|---|---|
| Control | Haskap | Difference (95% CI) | F | p-Value | Effect Size η2 | |
| Time to exhaustion; TTE (s) * | ||||||
| Pre | 481.6 ± 65.5 | 466.5 ± 87.3 | 20.0 (2.0–38.1) | 5.174 | 0.031 | 0.161 |
| Post | 484.5 ± 69.8 | 488.5 ± 98.3 | ||||
| HR max | ||||||
| Pre | 188 ± 11 | 185 ± 10 | 1.9 (−1.1–4.8) | 1.683 | 0.206 | 0.058 |
| Post | 189 ± 10 | 184 ± 11 | ||||
| RPE | ||||||
| Pre | 18.7 ± 1.2 | 18.7 ± 1.3 | 0.35 (−0.12–0.81) | 2.348 | 0.137 | 0.080 |
| Post | 18.9 ± 0.9 | 18.5 ± 1.4 | ||||
| Lactate (mmol/L) | ||||||
| Pre | 7.68 ± 1.98 | 7.10 ± 1.86 | 0.22 (−1.43–0.98) | 0.144 | 0.707 | 0.005 |
| Post | 7.42 ± 2.01 | 7.26 ± 1.99 | ||||
| Relative O2peak (mL/kg/min) | ||||||
| Pre | 53.2 ± 6.6 | 52.2 ± 4.8 | 0.7 (−2.11–0.69) | 1.096 | 0.304 | 0.039 |
| Post | 53.6 ± 6.7 | 53.4 ± 4.8 | ||||
| AbsoluteO2peak (mL) | ||||||
| Pre | 3956 ± 493 | 4175 ± 439 | 0.45 (−112.10–113.01) | 2.317 | 0.140 | 0.79 |
| Post | 3968 ± 467 | 4265 ± 463 | ||||
| ANCOVA Adjusted for Baseline | ||||||
|---|---|---|---|---|---|---|
| Control | Haskap | Difference (95% CI) | F | p-Value | Effect Size η2 | |
| Mean speed (km/h) * | ||||||
| Pre | 13.33 ± 2.06 | 14.01 ± 1.62 | 0.25 (0.12–0.38) | 15.162 | 0.001 | 0.387 |
| Post | 13.27 ± 2.08 | 14.21 ± 1.66 | ||||
| Time (s) * | ||||||
| Pre | 1377 ± 192 | 1299 ± 141 | 20.9 (4.2–37.7) | 6.662 | 0.016 | 0.217 |
| Post | 1384 ± 193 | 1282 ± 140 | ||||
| RPE | ||||||
| Pre | 18.2 ± 0.7 | 18.3 ± 1.0 | 0.31 (−0.22–0.84) | 1.402 | 0.248 | 0.055 |
| Post | 18.5 ± 1.0 | 18.3 ± 1.1 | ||||
| Lactate (mmol/L) | ||||||
| Pre | 4.95 ± 1.57 | 6.49 ± 1.93 | 0.12 (−0.88–1.12) | 0.062 | 0.805 | 0.003 |
| Post | 5.48 ± 1.71 | 6.27 ± 1.37 | ||||
| Maximum HR (bpm) | ||||||
| Pre | 186 ± 10 | 186 ± 14 | 0.3 (−2.6–3.3) | 0.054 | 0.818 | 0.002 |
| Post | 186 ± 10 | 186 ± 13 | ||||
| Mean HR (bpm) | ||||||
| Pre | 177 ± 13 | 178 ± 13 | 0.19 (−0.31–3.34) | 0.015 | 0.905 | 0.001 |
| Post | 175 ± 12 | 176 ± 12 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howatson, G.; Snaith, G.C.; Kimble, R.; Cowper, G.; Keane, K.M. Improved Endurance Running Performance Following Haskap Berry (Lonicera caerulea L.) Ingestion. Nutrients 2022, 14, 780. https://doi.org/10.3390/nu14040780
Howatson G, Snaith GC, Kimble R, Cowper G, Keane KM. Improved Endurance Running Performance Following Haskap Berry (Lonicera caerulea L.) Ingestion. Nutrients. 2022; 14(4):780. https://doi.org/10.3390/nu14040780
Chicago/Turabian StyleHowatson, Glyn, Gemma C. Snaith, Rachel Kimble, Gavin Cowper, and Karen M. Keane. 2022. "Improved Endurance Running Performance Following Haskap Berry (Lonicera caerulea L.) Ingestion" Nutrients 14, no. 4: 780. https://doi.org/10.3390/nu14040780
APA StyleHowatson, G., Snaith, G. C., Kimble, R., Cowper, G., & Keane, K. M. (2022). Improved Endurance Running Performance Following Haskap Berry (Lonicera caerulea L.) Ingestion. Nutrients, 14(4), 780. https://doi.org/10.3390/nu14040780