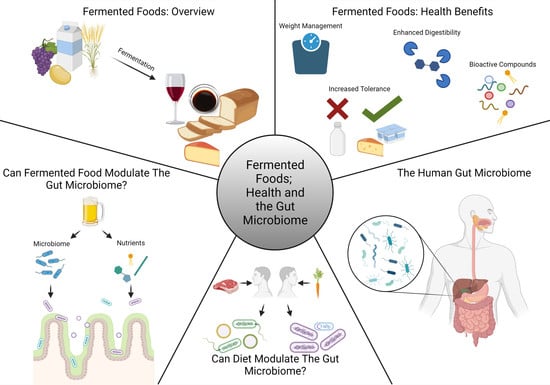
Fermented Foods, Health and the Gut Microbiome
Abstract
:1. Introduction
2. Fermented Food
2.1. Diversity of Fermented Food Types
2.2. Primary Food Fermentation Pathways
2.3. Microbiome of Fermented Foods
3. Health Benefits of Fermented Foods
3.1. Human Dietary Studies
3.2. Transformations in Food Arising from Fermentation
3.3. Release of Bioactive Peptides
3.4. Production of Exopolysaccharide
4. Evidence of Fermented Foods That Modulate the Gut Microbiome
5. Nutrients from Fermented Foods That Modulate the Gut Microbiome
6. Potential of Fermented Food Microbiota to Survive and Modulate the Gut Microbiome
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nunez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.-S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitorino, L.C.; Bessa, L.A. Technological microbiology: Development and applications. Front. Microbiol. 2017, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Macori, G.; Cotter, P.D. Novel insights into the microbiology of fermented dairy foods. Curr. Opin. Biotechnol. 2018, 49, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of fermented foods in food guides around the world. Nutrients 2015, 7, 390–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies; Heinemann: London, UK, 1907. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Probiotics in food; health and nutritional properties and guidelines for evaluation. In FAO Food and Nutrition Paper; FAO/WHO: Rome, Italy, 2006; p. 85. [Google Scholar]
- Gilliland, S.E.; Morelli, L.; Reid, G. Health and nutritional properties of probiotics in food including powder milk with live Lactic Acid Bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Shimizu, T. Health claims on functional foods: The Japanese regulations and an international comparison. Nutr. Res. Rev. 2003, 16, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diplock, A.T.; Aggett, P.J.; Ashwell, M.; Bornet, F.; Fern, E.B.; Roberfroid, M.B. Scientific concepts of functional foods in Europe consensus document. Br. J. Nutr. 1999, 81, S1–S27. [Google Scholar] [CrossRef] [Green Version]
- Kindstedt, P.S. Cheese and Culture: A History of Cheese and Its Place in Western Civilization, 1st ed.; Chelsea Green Publishing Company: Hartford, VT, USA, 2012; ISBN 9781603585064. [Google Scholar]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative foods: Microbiology, biochemistry, potential human health benefits and public health issues. Foods 2021, 10, 69. [Google Scholar] [CrossRef]
- Tamang, J.P. Diversity of Fermented Foods. In Fermented Foods and Beverages of the World, 1st ed.; Tamang, J.P., Kailasapathy, K., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 41–84. [Google Scholar] [CrossRef]
- Beresford, T.P.; Fitzsimons, N.A.; Brennan, N.L.; Cogan, T.M. Recent advances in cheese microbiology. Int. Dairy J. 2001, 11, 259–274. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madigan, M.T.; Martinko, J.M.; Dunlap, P.V.; Clark, D.P. Metabolic diversity: Catabolism of organic compounds. In Brock Biology of Microorganisms, 12th ed.; Berriman, E.W., Carlson, L., Hutchinson, G., Reed, E., Eds.; Pearson Education Incorporated: New York, NY, USA, 2009; pp. 612–651. [Google Scholar]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Eram, M.S.; Ma, K. Decarboxylation of pyruvate to acetaldehyde for ethanol production by hyperthermophiles. Biomolecules 2013, 3, 578–596. [Google Scholar] [CrossRef] [Green Version]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of acetic acid bacteria and their role in vinegar and fermented beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Garcia, R.A.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.K.; Marcellin, E. Microbial propionic acid production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Lasik-Kurdys, M.; Majcher, M.; Nowak, J. Effects of different techniques of malolactic fermentation induction on diacetyl metabolism and biosynthesis of selected aromatic esters in cool- climate grape wines. Molecules 2018, 23, 2549. [Google Scholar] [CrossRef] [Green Version]
- Beermann, C.; Hartung, J. Physiological properties of milk ingredients released by fermentation. Food Funct. 2013, 4, 185–199. [Google Scholar] [CrossRef]
- Müller, V. Bacterial fermentation. In Encyclopedia of Life Scicience; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar] [CrossRef]
- Tamang, J.P. Biochemical and modern identification techniques: Microfloras of fermented foods. In Encyclopedia Food Microbiolology, 2nd ed.; Batt, C., Patel, P., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 250–258. [Google Scholar] [CrossRef]
- Walker, G.; Stewart, G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Escalante, A.; Giles-Gómez, M.; Hernández, G.; Córdova-Aguilar, M.S.; López-Munguía, A.; Gosset, G.; Bolívar, F. Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int. J. Food. Microbiol. 2008, 124, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.L. Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: A neglected food group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Raspor, P.; Goranovič, D. Biotechnological applications of acetic acid bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Saichana, N.; Matsushita, K.; Adachi, O.; Frébort, I.; Frebortova, J. Acetic acid bacteria: A group of bacteria with versatile biotechnological applications. Biotechnol. Adv. 2015, 33, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Laëtitia, G.; Pascal, D.; Yann, D. The citrate metabolism in homo- and heterofermentative LAB: A selective means of becoming dominant over other microorganisms in complex ecosystems. Food Nutr. Sci. 2014, 5, 953–969. [Google Scholar] [CrossRef] [Green Version]
- Quintans, N.G.; Blancato, V.; Repizo, G.; Magni, C.; Lopez, P. Citrate metabolism and aroma compound production in lactic acid bacteria. Mol. Asp. Lact. Acid Bact. Tradit. New Appl. 2008, 37, 65–88. [Google Scholar]
- Betteridge, A.L.; Sumby, K.M.; Sundstrom, J.F.; Grbin, P.R.; Jiranek, V. Application of directed evolution to develop ethanol tolerant Oenococcus oeni for more efficient malolactic fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 921–932. [Google Scholar] [CrossRef]
- Davis, C.R.; Wibowo, D.; Eschenbruch, R.; Lee, T.H.; Fleet, G.H. Practical implications of malolactic fermentation: A review. Am. J. Enol. Vitic. 1985, 36, 290–301. [Google Scholar]
- Kunkee, R.E. Some roles of malic acid in the malolactic fermentation in wine making. FEMS Microbiol. Lett. 1991, 88, 55–71. [Google Scholar] [CrossRef]
- Liu, S.Q. Malolactic fermentation in wine-beyond deacidification. J. Appl. Microbiol. 2002, 92, 589–601. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Arendt, E.; Hill, C.; Stanton, C.; Ross, R.P. Recent advances in microbial fermentation for dairy and health. F1000Research 2017, 6, 751. [Google Scholar] [CrossRef] [PubMed]
- Coeuret, V.; Dubernet, S.; Bernardeau, M.; Gueguen, M.; Vernoux, J.P. Isolation, characterisation and identification of lactobacilli focusing mainly on cheeses and other dairy products. Lait 2003, 83, 269–306. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. High-throughput sequencing for detection of subpopulations of bacteria not previously associated with artisanal cheeses. Appl. Environ. Microbiol. 2012, 78, 5717–5723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamang, J.P.; Holzapfel, W.H.; Shin, D.H.; Felis, G.E. Editorial: Microbiology of ethnic fermented foods and alcoholic beverages of the world. Front. Microbiol. 2017, 8, 1377. [Google Scholar] [CrossRef] [Green Version]
- Cotter, P.D.; Beresford, T.P. Microbiome changes during ripening. In Cheese, Chemistry, Physics & Microbiology, 4th ed.; McSweeney, P.H.L., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: London, UK, 2017; Volume 1, pp. 389–409. [Google Scholar]
- Bittante, G.; Amalfitano, N.; Bergamaschi, M.; Patel, N.; Haddi, M.L.; Benabid, H.; Pazzola, M.; Vacca, G.M.; Tagliapietra, F.; Schiavon, S. Composition and aptitude for cheese-making of milk from cows, buffaloes, goats, sheep, dromedary camels, and donkeys. J. Dairy Sci. 2022, 105, 2132–2152. [Google Scholar] [CrossRef]
- Nikoloudaki, O.; Lemos Junior, W.J.F.; Borruso, L.; Campanaro, S.; De Angelis, M.; Vogel, R.F.; Di Cagno, R.; Gobbetti, M. How multiple farming conditions correlate with the composition of the raw cow’s milk lactic microbiome. Environ. Microbiol. 2021, 23, 1702–1716. [Google Scholar] [CrossRef]
- Salazar, J.K.; Gonsalves, L.J.; Fay, M.; Ramachandran, P.; Schill, K.M.; Tortorello, M.L. Metataxonomic profiling of native and starter microbiota during ripening of gouda cheese made with Listeria monocytogenes-contaminated unpasteurized milk. Front. Microbiol. 2021, 12, 642789. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wang, Y.; You, C.; Ren, J.; Chen, W.; Zheng, H.; Liu, Z. Variation in raw milk microbiota throughout 12 months and the impact of weather conditions. Sci. Rep. 2018, 8, 2371. [Google Scholar] [CrossRef] [Green Version]
- Rahmeh, R.; Akbar, A.; Kishk, M.; Al-Onaizi, T.; Al-Azmi, A.; Al-Shatti, A.; Shajan, A.; Al-Mutairi, S.; Akbar, B. Distribution and antimicrobial activity of lactic acid bacteria from raw camel milk. New Microbes New Infect. 2019, 30, 100560. [Google Scholar] [CrossRef]
- Lane, C.N.; Fox, P.F. Contribution of starter and adjunct lactobacilli to proteolysis in Cheddar cheese during ripening. Int. Dairy J. 1996, 6, 715–728. [Google Scholar] [CrossRef]
- González-González, F.; Delgado, S.; Ruiz, L.; Margolles, A.; Ruas-Madiedo, P. Functional bacterial cultures for dairy applications: Towards improving safety, quality, nutritional and health benefit aspects. J. Appl. Microbiol. 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- Bintsis, T. Yeasts in different types of cheese. AIMS Microbiol. 2021, 7, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Blaya, J.; Barzideh, Z.; LaPointe, G. Symposium review: Interaction of starter cultures and nonstarter lactic acid bacteria in the cheese environment. J. Dairy Sci. 2018, 101, 3611–3629. [Google Scholar] [CrossRef] [PubMed]
- Mounier, J.; Coton, M. Smear-ripened cheeses. In Encyclopedia of Dairy Science, 2nd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 343–351. [Google Scholar] [CrossRef]
- Ritschard, J.S.; Amato, L.; Kumar, Y.; Müller, B.; Meile, L.; Schuppler, M. The role of the surface smear microbiome in the development of defective smear on surface-ripened red-smear cheese. AIMS Microbiol. 2018, 4, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Hill, P.J.; Dodd, C.E.R. Bacterial community structure and location in Stilton cheese. Appl. Environ. Microbiol. 2003, 69, 3540–3548. [Google Scholar] [CrossRef] [Green Version]
- Gkatzionis, K.; Yunita, D.; Linforth, R.S.T.; Dickinson, M.; Dodd, C.E.R. Diversity and activities of yeasts from different parts of a Stilton cheese. Int. J. Food Microbiol. 2014, 177, 109–116. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Sun, Q.; Giovannucci, E.; Mozaffarian, D.; Manson, J.A.E.; Willett, W.C.; Hu, F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014, 12, 215. [Google Scholar] [CrossRef] [Green Version]
- Eussen, S.J.P.M.; Van Dongen, M.C.J.M.; Wijckmans, N.; Den Biggelaar, L.; Oude Elferink, S.J.W.H.; Singh-Povel, C.M.; Schram, M.T.; Sep, S.J.S.; van der Kallen, C.J.; Koster, A.; et al. Consumption of dairy foods in relation to impaired glucose metabolism and type 2 diabetes mellitus: The Maastricht Study. Br. J. Nutr. 2016, 115, 1453–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soedamah-Muthu, S.S.; Masset, G.; Verberne, L.; Geleijnse, J.M.; Brunner, E.J. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br. J. Nutr. 2013, 109, 718–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapsell, L.C. Fermented dairy food and CVD risk. Br. J. Nutr. 2015, 113, S131–S135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasa, M.; Aoi, W.; Mune, K.; Yamauchi, H.; Furuta, K.; Sasaki, S.; Takeda, K.; Harada, K.; Wada, S.; Nakamura, Y.; et al. Fermented milk improves glucose metabolism in exercise-induced muscle damage in young healthy men. Nutr. J. 2013, 12, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.Y.; Lee, M.S.; Jeon, J.Y.; Ha, E.S.; Kim, T.H.; Yoon, J.Y.; Ok, C.-O.; Lee, H.-K.; Hwang, W.-S.; Choe, S.J.; et al. Beneficial effects of fresh and fermented kimchi in prediabetic individuals. Ann. Nutr. Metab. 2013, 63, 111–119. [Google Scholar] [CrossRef]
- Han, K.; Bose, S.; Wang, J.H.; Kim, B.S.; Kim, M.J.; Kim, E.J.; Kim, H. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Mol. Nutr. Food Res. 2015, 59, 1004–1008. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401. [Google Scholar] [CrossRef] [Green Version]
- Hilimire, M.R.; DeVylder, J.E.; Forestell, C.A. Fermented foods, neuroticism, and social anxiety: An interaction model. Psychiatry Res. 2015, 228, 203–208. [Google Scholar] [CrossRef]
- Omagari, K.; Sakaki, M.; Tsujimoto, Y.; Shiogama, Y.; Iwanaga, A.; Ishimoto, M.; Yamaguchi, A.; Masuzumi, M.; Kawase, M.; Ichimura, M.; et al. Coffee consumption is inversely associated with depressive status in Japanese patients with type 2 diabetes. J. Clin. Biochem. Nutr. 2014, 55, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S.; et al. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems 2020, 5, 901–920. [Google Scholar] [CrossRef] [Green Version]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Impact of fermented foods on human cognitive function—A review of outcome of clinical trials. Sci. Pharm. 2018, 86, 22. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Khetarpaul, N. Indigenous legume fermentation: Effect on some antinutrients and in-vitro digestibility of starch and protein. Food Chem. 1994, 50, 403–406. [Google Scholar] [CrossRef]
- Çabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of fermentation on the protein digestibility and levels of non-nutritive compounds of pea protein concentrate. Food Technol. Biotechnol. 2018, 56, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Jardin, J.; Mollé, D.; Piot, M.; Lortal, S.; Gagnaire, V. Quantitative proteomic analysis of bacterial enzymes released in cheese during ripening. Int. J. Food Microbiol. 2012, 155, 19–28. [Google Scholar] [CrossRef]
- Rozenberg, S.; Body, J.J.; Bruyere, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.P.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; et al. Effects of dairy products consumption on health: Benefits and beliefs—A commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2015, 98, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Hong, K.-J.; Lee, C.-H.; Kim, S.W. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food 2004, 7, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Fermented food in the context of a healthy diet: How to produce novel functional foods? Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 574–581. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. The role of fermentation in providing biologically active compounds for the human organism. In Fermentation: Effects on Food Properties; Metha, B.M., Kamal-Eldin, A., Iwanski, R.Z., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 151–168. [Google Scholar]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2017, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Hudson, J.A.; Korpela, R.; de los Reyes-Gavilán, C.G. Impact on human health of microorganisms present in fermented dairy products: An overview. BioMed Res. Int. 2015, 2015, 412714. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.; Ross, R.P.; Fitzgerald, G.F.; Van Sinderen, D. Fermented functional foods based on probiotics and their biogenic metabolites. Curr. Opin. Biotechnol. 2005, 16, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Altay, F.; Karbancioglu-Güler, F.; Daskaya-Dikmen, C.; Heperkan, D. A review on traditional Turkish fermented non-alcoholic beverages: Microbiota, fermentation process and quality characteristics. Int. J. Food Microbiol. 2013, 167, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Noh, J.S.; Song, Y.O. Beneficial effects of kimchi, a Korean fermented vegetable food, on pathophysiological factors related to atherosclerosis. J. Med. Food 2017, 21, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Solieri, L.; Rutella, G.S.; Tagliazucchi, D. Impact of non-starter lactobacilli on release of peptides with angiotensin-converting enzyme inhibitory and antioxidant activities during bovine milk fermentation. Food Microbiol. 2015, 51, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, A.K.; Sanjukta, S.; Jeyaram, K. Production of angiotensin I converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension. Crit. Rev. Food Sci. Nutr. 2017, 57, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Xue, J.; Yang, J.; Chen, X.; Shao, Y.; Kwok, L.-Y.; Bilige, M.; Mang, L.; Zhang, H. Angiotensin-converting enzyme inhibitory activity of Lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. J. Dairy Sci. 2014, 97, 6680–6692. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Gerocarni, B.; Laghi, L.; Borghi, C. Blood pressure lowering effect of lactotripeptides assumed as functional foods: A meta-analysis of current available clinical trials. J. Hum. Hypertens. 2011, 25, 425–436. [Google Scholar] [CrossRef] [Green Version]
- Takeda, S.; Matsufuji, H.; Nakade, K.; Takenoyama, S.I.; Ahhmed, A.; Sakata, R.; Kawahara, S.; Muguruma, M. Investigation of lactic acid bacterial strains for meat fermentation and the product’s antioxidant and angiotensin-I-converting-enzyme inhibitory activities. Anim. Sci. J. 2017, 88, 507–516. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef]
- Ryan, P.M.; Ross, R.P.; Fitzgerald, G.F.; Caplice, N.M.; Stanton, C. Sugar-coated: Exopolysaccharide producing lactic acid bacteria for food and human health applications. Food Funct. 2015, 6, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Tok, E.; Aslim, B. Cholesterol removal by some lactic acid bacteria that can be used as probiotic. Microbiol. Immunol. 2010, 54, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, R.M.; Cuesta, E.P.; Gilliland, S.E. Binding of free bile acids by cells of yogurt starter culture bacteria. J. Dairy Sci. 2002, 85, 2705–2710. [Google Scholar] [CrossRef] [Green Version]
- London, L.E.; Kumar, A.H.; Wall, R.; Casey, P.G.; O’Sullivan, O.; Shanahan, F.; Hill, C.; Cotter, P.D.; Fitzgerald, G.F.; Ross, R.P.; et al. Exopolysaccharide-producing probiotic lactobacilli reduce serum cholesterol and modify enteric microbiota in ApoE-deficient mice. J. Nutr. 2014, 144, 1956–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mårtensson, O.; Biörklund, M.; Lambo, A.M.; Dueñas-Chasco, M.; Irastorza, A.; Holst, O.; Norin, E.; Welling, G.; Öste, R.; Önning, G. Fermented, ropy, oat-based products reduce cholesterol levels and stimulate the bifidobacteria flora in humans. Nutr. Res. 2005, 25, 429–442. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Veiga, P.; Pons, N.; Agrawal, A.; Oozeer, R.; Guyonnet, D.; Brazeilles, R.; Faurie, J.M.; van Hylckama Vlieg, J.E.; Houghton, L.A.; Whorwell, P.J.; et al. Changes of the human gut microbiome induced by a fermented milk product. Sci. Rep. 2014, 4, 6328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unno, T.; Choi, J.H.; Hur, H.G.; Sadowsky, M.J.; Ahn, Y.T.; Huh, C.S.; Kim, G.B.; Cha, C.J. Changes in human gut microbiota influenced by probiotic fermented milk ingestion. J. Dairy Sci. 2015, 98, 3568–3576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yılmaz, İ.; Enver Dolar, M.; Özpınar, H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: A randomized controlled trial. Turk. J. Gastroenterol. 2019, 30, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Jia, H.; Zhang, X.; Wang, X.; Wang, Z.; Gao, Z.; Yuan, Y.; Yue, T. Supplementation of kefir ameliorates azoxymethane/dextran sulfate sodium induced colorectal cancer by modulating the gut microbiota. Food Funct. 2021, 12, 11641–11655. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Sheu, B.S. Probiotics-containing yogurts suppress Helicobacter pylori load and modify immune response and intestinal microbiota in the Helicobacter pylori-infected children. Helicobacter 2012, 17, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Lisko, D.; Johnston, G.; Johnston, C. Effects of dietary yogurt on the healthy human gastrointestinal (GI) microbiome. Microorganisms 2017, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Firmesse, O.; Rabot, S.; Bermúdez-Humarán, L.G.; Corthier, G.; Furet, J.P. Consumption of Camembert cheese stimulates commensal enterococci in healthy human intestinal microbiota. FEMS Microbiol. Lett. 2007, 276, 189–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firmesse, O.; Alvaro, E.; Mogenet, A.; Bresson, J.L.; Lemée, R.; Le Ruyet, P.; Bonhomme, C.; Lambert, D.; Andrieux, C.; Doré, J.; et al. Fate and effects of Camembert cheese micro-organisms in the human colonic microbiota of healthy volunteers after regular Camembert consumption. Int. J. Food Microbiol. 2008, 125, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, C.I.; Kurilshikov, A.; Leeming, E.R.; Visconti, A.; Bowyer, R.C.E.; Menni, C.; Fachi, M.; Koutnikova, H.; Veiga, P.; Zhernakova, A.; et al. Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; Krych, L.; Nielsen, D.S. Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation—A pilot study. Food Funct. 2018, 9, 5323–5335. [Google Scholar] [CrossRef]
- Inoguchi, S.; Ohashi, Y.; Narai-Kanayama, A.; Aso, K.; Nakagaki, T.; Fujisawa, T. Effects of non-fermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans. Int. J. Food Sci. Nutr. 2012, 63, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.C.; Shang, H.F.; Lin, T.F.; Wang, T.H.; Lin, H.S.; Lin, S.H. Effect of fermented soy milk on the intestinal bacterial ecosystem. World J. Gastroenterol. 2005, 11, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, B.; Suzuki, Y.; Yonezu, T.; Mizushima, N.; Watanabe, N.; Sato, T.; Inoue, S.; Inokuchi, S. Cha-Koji, comprising green tea leaves fermented with Aspergillus luchuensis var kawachii kitahara, increases regulatory T cell production in mice and humans. Biosci. Biotechnol. Biochem. 2018, 82, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Chen, Y.J.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.-K. Regulatory efficacy of fermented plant extract on the intestinal microflora and lipid profile in mildly hypercholesterolemic individuals. J. Food Drug. Anal. 2017, 25, 819–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Chu, X.; Cheng, Y.; Tang, S.; Zogona, D.; Pan, S.; Xu, X. Modulation of gut microbiota by lactobacillus casei fermented raspberry juice in vitro and in vivo. Foods 2021, 10, 3055. [Google Scholar] [CrossRef]
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014, 5, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Shiferaw Terefe, N.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food. Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of foods and beverages as a tool for increasing availability of bioactive compounds. Focus on short-chain fatty acids. Foods 2020, 9, 999. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Zheng, C.J.; Liu, R.; Xue, B.; Luo, J.; Gao, L.; Wang, Y.; Ou, S.; Li, S.; Peng, X. Impact and consequences of polyphenols and fructooligosaccharide interplay on gut microbiota in rats. Food Funct. 2017, 8, 1925–1932. [Google Scholar] [CrossRef]
- Gan, R.Y.; Shah, N.P.; Wang, M.F.; Lui, W.Y.; Corke, H. Fermentation alters antioxidant capacity and polyphenol distribution in selected edible legumes. Int. J. Food Sci. Technol. 2016, 51, 875–884. [Google Scholar] [CrossRef]
- Zhai, F.H.; Liu, H.Y.; Han, J.R. Protein nutritional value, polyphenols and antioxidant properties of corn fermented with Agaricus brasiliensis and Agaricus bisporus. World J. Microbiol. Biotechnol. 2018, 34, 36. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hervert-Hernández, D.; Goñi, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Y.L.; Zhang, X.; Wang, K.B.; Huang, J.A.; Liu, Z.H.; Zhu, M.-Z. Polyphenols from Fu Brick tea reduce obesity via modulation of gut microbiota and gut microbiota-related intestinal oxidative stress and barrier function. J. Agric. Food Chem. 2021, 69, 14530–14543. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef]
- Aura, A.M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Champ, C.E.; Kundu-Champ, A. Maximizing polyphenol content to uncork the relationship between wine and cancer. Front. Nutr. 2019, 6, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Postigo, M.; Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Coin-Araguez, L.; Del Mar Roca-Rodriguez, M.; Delgado-Lista, J.; Cardona, F.; Andres-Lacueva, C.; Tinahones, F.J. Effect of acute and chronic red wine consumption on lipopolysaccharide concentrations. Am. J. Clin. Nutr. 2013, 97, 1053–1061. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [Green Version]
- Barroso, E.; Muñoz-González, I.; Jiménez, E.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; del Carmen Martínez-Cuesta, M.; Requena, T. Phylogenetic profile of gut microbiota in healthy adults after moderate intake of red wine. Mol. Nutr. Food Res. 2017, 61, 1600620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; Van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tassell, M.L.; Miller, M.J. Lactobacillus Adhesion to Mucus. Nutrients 2011, 3, 613–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicard, J.-F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Meguro, S.; Hase, T.; Tokimitsu, I.; Sakata, T. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp. Biochem. Physiol.—A Mol. Integr. Physiol. 2000, 125, 525–531. [Google Scholar] [CrossRef]
- Willemsen, L.E.M.; Koetsier, M.A.; Van Deventer, S.J.H.; Van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hylckama Vlieg, J.E.T.; Veiga, P.; Zhang, C.; Derrien, M.; Zhao, L. Impact of microbial transformation of food on health-from fermented foods to fermentation in the gastro-intestinal tract. Curr. Opin. Biotechnol. 2011, 22, 211–219. [Google Scholar] [CrossRef]
- Darzi, J.; Frost, G.S.; Montaser, R.; Yap, J.; Robertson, M.D. Influence of the tolerability of vinegar as an oral source of short-chain fatty acids on appetite control and food intake. Int. J. Obes. 2014, 38, 675–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuente, M.A.; Fontecha, J.; Juárez, M. Fatty acid composition of the triglyceride and free fatty acid fractions in different cows-, ewes- and goats-milk cheeses. Z. Lebensm. Unters. Forsch. 2005, 196, 155–158. [Google Scholar] [CrossRef]
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Summer, A.; Formaggioni, P.; Franceschi, P.; Di Frangia, F.; Righi, F.; Malacarne, M. Cheese as functional food: The example of parmigiano reggiano and grana padano. Food Technol. Biotechnol. 2017, 55, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Moslemy, M.; Fard, R.M.N.; Hosseini, S.M.; Homayouni-Rad, A.; Mortazavian, A.M. Incorporation of propionibacteria in fermented milks as a probiotic. Crit. Rev. Food Sci. Nutr. 2016, 56, 1290–1312. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Bruneau, A.; Philippe, C.; Rochet, V.; Rouault, A.; Herve, C.; Roland, N.; Rabot, S.; Jan, G. Survival and metabolic activity of selected strains of Propionibacterium freudenreichii in the gastrointestinal tract of human microbiota-associated rats. Br. J. Nutr. 2007, 97, 714–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zago, M.; Fornasari, M.E.; Carminati, D.; Burns, P.; Suàrez, V.; Vinderola, G.; Reinheimer, J.; Giraffa, G. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 2011, 28, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Lactobacillus plantarum—Survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Haller, D.; Colbus, H.; Gänzle, M.G.; Scherenbacher, P.; Bode, C.; Hammes, W.P. Metabolic and functional properties of lactic acid bacteria in the gastro-intestinal ecosystem: A comparative in vitro study between bacteria of intestinal and fermented food origin. Syst. Appl. Microbiol. 2001, 24, 218–226. [Google Scholar] [CrossRef]
- Beganović, J.; Kos, B.; Leboš Pavunc, A.; Uroić, K.; Jokić, M.; Šušković, J. Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Prasad, D.N. Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int. J. Food Microbiol. 2005, 103, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. The potential of non-starter lactic acid bacteria from Cheddar cheese to colonise the gut. J. Funct. Foods 2021, 83, 104425. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Lonigro, S.L.; De Angelis, M.; Morelli, L.; Callegari, M.L.; Rizzello, C.G.; Visconti, A. Study of adhesion and survival of lactobacilli and bifidobacteria on table olives with the aim of formulating a new probiotic food. Appl. Environ. Microbiol. 2005, 71, 4233–4240. [Google Scholar] [CrossRef] [Green Version]
- Saxelin, M.; Lassig, A.; Karjalainen, H.; Tynkkynen, S.; Surakka, A.; Vapaatalo, H.; Järvenpää, S.; Korpela, R.; Mutanen, M.; Hatakka, K. Persistence of probiotic strains in the gastrointestinal tract when administered as capsules, yoghurt, or cheese. Int. J. Food Microbiol. 2010, 144, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M.D.; McMahon, D.J.; Broadbent, J.R. Comparative evaluation of yogurt and low-fat cheddar cheese as delivery media for probiotic Lactobacillus casei. J. Food Sci. 2008, 73, M375–M377. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.; Hayes, J.J.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Protection of Candidate Probiotic Lactobacilli by Cheddar Cheese Matrix during Simulated Gastrointestinal Digestion. J. Funct. Foods, 2022; in press. [Google Scholar]
- Mauro, C.; Guergoletto, K.; Garcia, S. Development of blueberry and carrot juice blend fermented by Lactobacillus reuteri LR92. Beverages 2016, 2, 37. [Google Scholar] [CrossRef] [Green Version]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Odamaki, T.; Kato, K.; Sugahara, H.; Xiao, J.Z.; Abe, F.; Benno, Y. Effect of probiotic yoghurt on animal-based diet-induced change in gut microbiota: An open, randomised, parallel-group study. Benef. Microbes 2016, 7, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; Van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The gut microbiome modulates colon tumorigenesis. mBio 2013, 4, e00692-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajilić-Stojanović, M.; Biagi, E.; Heilig, H.G.H.J.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Göker, M.; Gronow, S.; Zeytun, A.; Nolan, M.; Lucas, S.; Lapidus, A.; Hammon, N.; Deshpande, S.; Cheng, J.F.; Pitluck, S.; et al. Complete genome sequence of Odoribacter splanchnicus type strain (1651/6 T). Stand. Genom. Sci. 2011, 4, 200–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devkota, S.; Chang, E.B. Interactions between diet, bile acid metabolism, gut microbiota, and inflammatory bowel diseases. Dig. Dis. 2015, 33, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNulty, N.P.; Yatsunenko, T.; Hsiao, A.; Faith, J.J.; Muegge, B.D.; Goodman, A.L.; Henrissat, B.; Oozeer, R.; Cools-Portier, S.; Gobert, G.; et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci. Transl. Med. 2011, 3, 106ra106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The long-term stability of the human gut microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.M.; Jhoo, J.W.; Pak, J.I.; Kwon, I.K.; Lee, S.K.; Kim, G.Y. Effect of yogurt containing deep sea water on health-related serum parameters and intestinal microbiota in mice. J. Dairy Sci. 2015, 98, 5967–5973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plé, C.; Breton, J.; Daniel, C.; Foligné, B. Maintaining gut ecosystems for health: Are transitory food bugs stowaways or part of the crew? Int. J. Food Microbiol. 2015, 213, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.M.; Eisen, J.A.; Zivkovic, A.M. The microbes we eat: Abundance and taxonomy of microbes consumed in a day’s worth of meals for three diet types. PeerJ 2014, 2, 659. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Shin, D.H.; Jung, S.J.; Chae, S.W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whon, T.W.; Ahn, S.W.; Yang, S.; Kim, J.Y.; Kim, Y.B.; Kim, Y.; Hong, J.-M.; Jung, H.; Choi, Y.-E.; Lee, S.H.; et al. ODFM, an omics data resource from microorganisms associated with fermented foods. Sci. Data 2021, 8, 113. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Selhub, E.M.; Logan, A.C.; Bested, A.C. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. J. Physiol. Anthropol. 2014, 33, 2. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, F.; Vitaglione, P.; Cuomo, R.; Canani, R.B.; Ercolini, D. Dietary interventions to modulate the gut microbiome-how far away are we from precision medicine. Inflamm. Bowel Dis. 2018, 24, 2142–2154. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Laghi, L.; Gobbetti, M.; Ercolini, D. Unusual sub-genus associations of faecal Prevotella and Bacteroides with specific dietary patterns. Microbiome 2016, 4, 57. [Google Scholar] [CrossRef] [Green Version]
| Fermentation Type | Substrate | End Products | Microorganisms Responsible | Reference |
|---|---|---|---|---|
| Lactic Acid * | Sugar | |||
| Homo lactic | Lactic acid | Lactococcus lactis | [18] | |
| Streptococcus thermophilus | ||||
| Lactobacillus delbrueckii subsp. bulgaricus | ||||
| Lactobacillus acidophilus | ||||
| Lactobacillus helveticus | ||||
| Pediococcus | ||||
| Enterococcus | ||||
| Hetero lactic | Lactic acid, ethanol, CO2 | Leuconostoc | [18] | |
| Fructilactobacillus sanfranciscensis | ||||
| Levilactobacillus brevis | ||||
| Limosilactobacillus fermentum | ||||
| Limosilactobacillus reuteri | ||||
| Lacticaseibacillus casei | ||||
| Lactiplantibacillus plantarum | ||||
| Latilactobacillus curvatus | ||||
| Ethanol | Sugar | Ethanol, CO2 | Saccharomyces cerevisiae | [19] |
| Zymomonas mobilis | ||||
| Acetic Acid | Ethanol | Acetic acid | Acetobacter | [20] |
| Komagataeibacter | ||||
| Propionic Acid | Lactic Acid | Propionic acid, acetic acid, CO2 | Propionibacterium freudenreichii | [21] |
| Propionibacterium jensenii | ||||
| Propionibacterium thoenii | ||||
| Propionibacterium acidipropionici | ||||
| Propionibacterium cyclohexanicum | ||||
| Citric Acid | Citric Acid | Acetate, Formate, Ethanol, 2,3-butanediol, Diacetyl, Acetoin, CO2, Lactate | Lactococcus lactis subsp. lactis biovar diacetylactis | [22] |
| Leuconostoc ** | ||||
| Enterococcus ** | ||||
| Lactobacillus ** | ||||
| Oenococcus oeni | ||||
| Malolactic | Malic Acid | Lactic acid, CO2 | Oenococcus oeni | [23] |
| Lactobacillaceae *** | ||||
| Pediococcus ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. https://doi.org/10.3390/nu14071527
Leeuwendaal NK, Stanton C, O’Toole PW, Beresford TP. Fermented Foods, Health and the Gut Microbiome. Nutrients. 2022; 14(7):1527. https://doi.org/10.3390/nu14071527
Chicago/Turabian StyleLeeuwendaal, Natasha K., Catherine Stanton, Paul W. O’Toole, and Tom P. Beresford. 2022. "Fermented Foods, Health and the Gut Microbiome" Nutrients 14, no. 7: 1527. https://doi.org/10.3390/nu14071527
APA StyleLeeuwendaal, N. K., Stanton, C., O’Toole, P. W., & Beresford, T. P. (2022). Fermented Foods, Health and the Gut Microbiome. Nutrients, 14(7), 1527. https://doi.org/10.3390/nu14071527