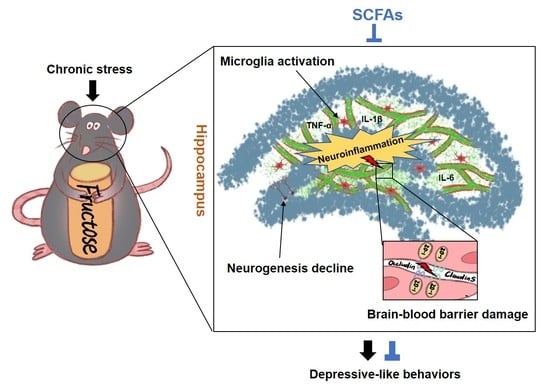
Short-Chain Fatty Acids Ameliorate Depressive-like Behaviors of High Fructose-Fed Mice by Rescuing Hippocampal Neurogenesis Decline and Blood–Brain Barrier Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Behavioral Tests
2.2.1. SPT
2.2.2. OFT
2.2.3. FST
2.2.4. TST
2.3. Blood Collection and Corticosterone Measurement
2.4. Immunofluorescence Staining
2.5. Dextran-FITC Treatment
2.6. Isolation of Brain Vasculature
2.7. Western Blotting
2.8. Statistics
3. Result
3.1. Depressive-like Behaviors Induced by Chronic Stress Are Aggravated in FruD Mice
3.2. Chronic Stress Worsens the Decline of Hippocampal Neurogenesis in FruD Mice
3.3. Chronic Stress Exacerbates the Damage of BBB Integrity and the Activation of Microglia in FruD Mice
3.4. SCFAs and Pioglitazone Ameliorate the Depressive-like Behaviors of FruD-CS Mice
3.5. SCFAs and Pioglitazone Rescue Hippocampal Neurogenesis Decline and BBB Damage in FruD-CS Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gore, F.M.; Bloem, P.J.N.; Patton, G.C.; Ferguson, J.; Joseph, V.; Coffey, C.; Sawyer, S.M.; Mathers, C.D. Global burden of disease in young people aged 10–24 years: A systematic analysis. Lancet 2011, 377, 2093–2102. [Google Scholar] [CrossRef]
- McCarron, R.M.; Shapiro, B.; Rawles, J.; Luo, J. Depression. Ann. Intern. Med. 2021, 174, ITC65–ITC80. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.; Probert, T.; Jones, E.; Young, A.H.; Robbins, I. Adapting to Stress: Understanding the Neurobiology of Resilience. Behav. Med. 2017, 43, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, D.F.; Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Li, Y.; Lv, M.-R.; Wei, Y.-J.; Sun, L.; Zhang, J.-X.; Zhang, H.-G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef]
- Khosravi, M.; Sotoudeh, G.; Majdzadeh, R.; Nejati, S.; Darabi, S.; Raisi, F.; Esmaillzadeh, A.; Sorayani, M. Healthy and unhealthy dietary patterns are related to depression: A case-control study. Psychiatry Investig. 2015, 12, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Cong, X.; Tracy, M.; Edmunds, L.S.; Hosler, A.S.; Appleton, A.A. The relationship between inflammatory dietary pattern in childhood and depression in early adulthood. Brain Behav. Immun. Health 2020, 2, 100017. [Google Scholar] [CrossRef]
- Herman, M.A.; Birnbaum, M.J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef]
- Chakraborti, A.; Graham, C.; Chehade, S.; Vashi, B.; Umfress, A.; Kurup, P.; Vickers, B.; Chen, H.A.; Telange, R.; Berryhill, T.; et al. High fructose corn syrup-moderate fat diet potentiates anxio-depressive behavior and alters ventral striatal neuronal signaling. Front. Neurosci. 2021, 15, 669410. [Google Scholar] [CrossRef]
- Harrell, C.S.; Burgado, J.; Kelly, S.D.; Johnson, Z.P.; Neigh, G.N. High-fructose diet during periadolescent development increases depressive-like behavior and remodels the hypothalamic transcriptome in male rats. Psychoneuroendocrinology 2015, 62, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Harrell, C.S.; Zainaldin, C.; McFarlane, D.; Hyer, M.M.; Stein, D.; Sayeed, I.; Neigh, G.N. High-fructose diet during adolescent development increases neuroinflammation and depressive-like behavior without exacerbating outcomes after stroke. Brain Behav. Immun. 2018, 73, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Kloster, A.; Hyer, M.M.; Dyer, S.; Salome-Sanchez, C.; Neigh, G.N. High fructose diet induces sex-specific modifications in synaptic respiration and affective-like behaviors in rats. Neuroscience 2021, 454, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.E.; Frigy, A.; Szasz, J.A.; Horvath, E. Neuroinflammation and microglia/macrophage phenotype modulate the molecular background of post-stroke depression: A literature review. Exp. Ther. Med. 2020, 20, 2510–2523. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.A.; Dion-Albert, L.; Lebel, M.; LeClair, K.; Labrecque, S.; Tuck, E.; Ferrer Perez, C.; Golden, S.A.; Tamminga, C.; Turecki, G.; et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc. Natl. Acad. Sci. USA 2020, 117, 3326–3336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sántha, P.; Veszelka, S.; Hoyk, Z.; Mészáros, M.; Walter, F.R.; Tóth, A.E.; Kiss, L.; Kincses, A.; Oláh, Z.; Seprényi, G.; et al. Restraint stress-induced morphological changes at the blood-brain barrier in adult rats. Front. Mol. Neurosci. 2015, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef]
- Anacker, C.; Luna, V.M.; Stevens, G.S.; Millette, A.; Shores, R.; Jimenez, J.C.; Chen, B.; Hen, R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 2018, 559, 98–102. [Google Scholar] [CrossRef]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional factors affecting adult neurogenesis and cognitive function. Adv. Nutr. 2017, 8, 804–811. [Google Scholar] [CrossRef]
- Li, J.-M.; Yu, R.; Zhang, L.-P.; Wen, S.-Y.; Wang, S.-J.; Zhang, X.-Y.; Xu, Q.; Kong, L.-D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Ju, Y.; Cui, L.L.; Liu, T.; Hou, Y.Y.; Wu, Q.; Ojo, O.; Du, X.J.; Wang, X.H. Association between dietary fiber intake and incidence of depression and anxiety in patients with essential hypertension. Nutrients 2021, 13, 4159. [Google Scholar] [CrossRef]
- Khayyatzadeh, S.S.; Omranzadeh, A.; Miri-Moghaddam, M.M.; Arekhi, S.; Naseri, A.; Ziaee, A.; Khajavi, L.; Salehkhani, F.N.; Ferns, G.A.; Ghayour-Mobarhan, M. Dietary antioxidants and fibre intake and depressive symptoms in Iranian adolescent girls. Public Health Nutr. 2021, 24, 5650–5656. [Google Scholar] [CrossRef] [PubMed]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanes, C.; Bittinger, K.; Gao, Y.; Friedman, E.S.; Nessel, L.; Paladhi, U.R.; Chau, L.; Panfen, E.; Fischbach, M.A.; Braun, J.; et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microb. 2021, 29, 394–407.e5. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Muller, B.; Rasmusson, A.J.; Just, D.; Jayarathna, S.; Moazzami, A.; Novicic, Z.K.; Cunningham, J.L. Fecal short-chain fatty acid ratios as related to gastrointestinal and depressive symptoms in young adults. Psychosom. Med. 2021, 83, 693–699. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. London 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Tsai, S.F.; Chen, P.C.; Kuo, Y.M.; Chen, Y.W. Pioglitazone rescues high-fat diet-induced depression-like phenotypes and hippocampal astrocytic deficits in mice. Biomed. Pharmacother. 2021, 140, 111734. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, X.; Yan, S.; Xie, X.; Fan, Y.; Zhang, J.; Peng, C.; You, Z. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARgamma-mediated alteration of microglial activation phenotypes. J. Neuroinflamm. 2016, 13, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.-Y.; He, J.-G.; Hu, Z.-L.; Xue, S.-G.; Xu, J.-F.; Cui, Q.-Q.; Gao, S.-Q.; Zhou, B.; Wu, P.-F.; Long, L.-H.; et al. A-kinase anchoring protein 150 and protein kinase a complex in the basolateral amygdala contributes to depressive-like behaviors induced by chronic restraint stress. Biol. Psychiatry 2019, 86, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhou, T.; Liao, L.; Yang, Z.; Wong, C.; Henn, F.; Malinow, R.; Yates, J.R.; Hu, H. βCaMKII in lateral habenula mediates core symptoms of depression. Science 2013, 341, 1016–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bales, K.R.; O’Neill, S.M.; Pozdnyakov, N.; Pan, F.; Caouette, D.; Pi, Y.Q.; Wood, K.M.; Volfson, D.; Cirrito, J.R.; Han, B.H.; et al. Passive immunotherapy targeting amyloid-beta reduces cerebral amyloid angiopathy and improves vascular reactivity. Brain 2016, 139, 563–577. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; Laplant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Pena, C.J.; Nestler, E.J.; Bagot, R.C. Environmental programming of susceptibility and resilience to stress in adulthood in male mice. Front. Behav. Neurosci. 2019, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.J.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014, 99, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Chocano-Bedoya, P.O.; O’Reilly, E.J.; Lucas, M.; Mirzaei, F.; Okereke, O.I.; Fung, T.T.; Hu, F.B.; Ascherio, A. Prospective study on long-term dietary patterns and incident depression in middle-aged and older women. Am. J. Clin. Nutr. 2013, 98, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Gururajan, A.; van de Wouw, M.; Boehme, M.; Becker, T.; O’Connor, R.; Bastiaanssen, T.F.S.; Moloney, G.M.; Lyte, J.M.; Silva, A.P.V.; Merckx, B.; et al. Resilience to chronic stress is associated with specific neurobiological, neuroendocrine and immune responses. Brain Behav. Immun. 2019, 80, 583–594. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, L.; Zeng, X.; Zhang, X.; Liu, Y.; Wu, Z.; Weng, P. The intervention of unique plant polysaccharides-Dietary fiber on depression from the gut-brain axis. Int. J. Biol. Macromol. 2021, 170, 336–342. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of stress in the brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Lee, H.J.; Welday, A.C.; Song, E.; Cho, J.; Sharp, P.E.; Jung, M.W.; Blair, H.T. Stress-induced alterations in hippocampal plasticity, place cells, and spatial memory. Proc. Natl. Acad. Sci. USA 2007, 104, 18297–18302. [Google Scholar] [CrossRef] [Green Version]
- Schoenfeld, T.J.; Gould, E. Stress, stress hormones, and adult neurogenesis. Exp. Neurol. 2012, 233, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Choe, S.; Woo, H.; Jeong, H.; An, H.K.; Moon, H.; Ryu, H.Y.; Yeo, B.K.; Lee, Y.W.; Choi, H.; et al. Autophagic death of neural stem cells mediates chronic stress-induced decline of adult hippocampal neurogenesis and cognitive deficits. Autophagy 2020, 16, 512–530. [Google Scholar] [CrossRef] [Green Version]
- Vinuesa, A.; Bentivegna, M.; Calfa, G.; Filipello, F.; Pomilio, C.; Bonaventura, M.M.; Lux-Lantos, V.; Matzkin, M.E.; Gregosa, A.; Presa, J.; et al. Early exposure to a high-fat diet impacts on hippocampal plasticity: Implication of microglia-derived exosome-like extracellular vesicles. Mol. Neurobiol. 2019, 56, 5075–5094. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yin, Y.; Du, L. Blood-Brain Barrier Dysfunction in the Pathogenesis of Major Depressive Disorder. Cell Mol. Neurobiol. 2021. [Google Scholar] [CrossRef]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [Green Version]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb Blood Flow Metab. 2020, 40, S6–S24. [Google Scholar] [CrossRef]
- Oldendorf, W.H. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am. J. Physiol. 1973, 224, 1450–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-F.; Wang, C.-Y.; Wang, J.-H.; Wang, Q.-N.; Li, S.-J.; Wang, H.-O.; Zhou, F.; Li, J.-M. Short-Chain Fatty Acids Ameliorate Depressive-like Behaviors of High Fructose-Fed Mice by Rescuing Hippocampal Neurogenesis Decline and Blood–Brain Barrier Damage. Nutrients 2022, 14, 1882. https://doi.org/10.3390/nu14091882
Tang C-F, Wang C-Y, Wang J-H, Wang Q-N, Li S-J, Wang H-O, Zhou F, Li J-M. Short-Chain Fatty Acids Ameliorate Depressive-like Behaviors of High Fructose-Fed Mice by Rescuing Hippocampal Neurogenesis Decline and Blood–Brain Barrier Damage. Nutrients. 2022; 14(9):1882. https://doi.org/10.3390/nu14091882
Chicago/Turabian StyleTang, Chuan-Feng, Cong-Ying Wang, Jun-Han Wang, Qiao-Na Wang, Shen-Jie Li, Hai-Ou Wang, Feng Zhou, and Jian-Mei Li. 2022. "Short-Chain Fatty Acids Ameliorate Depressive-like Behaviors of High Fructose-Fed Mice by Rescuing Hippocampal Neurogenesis Decline and Blood–Brain Barrier Damage" Nutrients 14, no. 9: 1882. https://doi.org/10.3390/nu14091882
APA StyleTang, C. -F., Wang, C. -Y., Wang, J. -H., Wang, Q. -N., Li, S. -J., Wang, H. -O., Zhou, F., & Li, J. -M. (2022). Short-Chain Fatty Acids Ameliorate Depressive-like Behaviors of High Fructose-Fed Mice by Rescuing Hippocampal Neurogenesis Decline and Blood–Brain Barrier Damage. Nutrients, 14(9), 1882. https://doi.org/10.3390/nu14091882