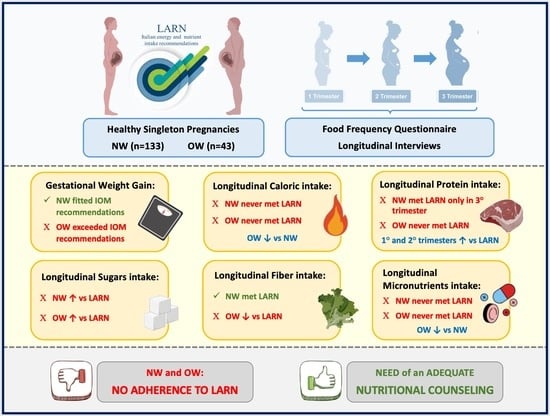
Longitudinal Nutritional Intakes in Italian Pregnant Women in Comparison with National Nutritional Guidelines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Dietary Intake Assessment
2.4. Dietary Bromatological Analysis
2.5. Energy and Protein References: Italian LARN
2.6. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Energy, Macro- and Micronutrients Intakes
3.3. Comparison with Recommendations
4. Discussion
4.1. Macronutrients and Fiber Intake
4.2. Micronutrients Intake
4.3. Strenghts and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NW | Normal Weight |
| OW | Over Weight |
| IOM | Institute of Medicine |
| PUFA | Polyunsatured Fatty Acids |
| GWG | Gestational Weight Gain |
| FFQ | Food Frequency Questionnaire |
| LARN | Livelli di Assunzione di Riferimento di Nutrienti ed energia |
| GA | Gestational Age |
| BMI | Body Mass Index |
| EIP | Energy Intake in Pregnancy |
| PIP | Protein Intake in Pregnancy |
| PAL | Physical Activity Level |
| EIF | Energy Intake for Female |
| PIF | Protein Intake for Female |
| ECP | Energy Cost of Pregnancy |
| PCP | Protein Cost of Pregnancy |
References
- Kaiser, L.; Allen, L.H.; Holt, K.; Polly, D.; Stang, J. Position of the American Dietetic Association: Nutrition and lifestyle for a healthy pregnancy outcome. J. Am. Diet. Assoc. 2010, 110, 141. [Google Scholar] [CrossRef]
- Danielewicz, H.; Myszczyszyn, G.; Dębińska, A.; Myszkal, A.; Boznański, A.; Hirnle, L. Diet in pregnancy-more than food. Eur. J. Pediatr. 2017, 176, 1573–1579. [Google Scholar] [CrossRef] [Green Version]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Cetin, I.; Bühling, K.; Demir, C.; Kortam, A.; Prescott, S.L.; Yamashiro, Y.; Yarmolinskaya, M.; Koletzko, B. Impact of Micronutrient Status during Pregnancy on Early Nutrition Programming. Ann. Nutr. Metab. 2019, 74, 269–278. [Google Scholar] [CrossRef]
- Berti, C.; Cetin, I.; Agostoni, C.; Desoye, G.; Devlieger, R.; Emmett, P.M.; Ensenauer, R.; Hauner, H.; Herrera, E.; Hoesli, I.; et al. Pregnancy and Infants’ Outcome: Nutritional and Metabolic Implications. Crit. Rev. Food Sci. Nutr. 2016, 56, 82–91. [Google Scholar] [CrossRef]
- Cetin, I.; Berti, C.; Calabrese, S. Role of micronutrients in the periconceptional period. Hum. Reprod. Update 2010, 16, 80–95. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of women’s nutrition before and during early pregnancy on maternal and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26, 285–301. [Google Scholar] [CrossRef]
- Palaniswamy, S.; Williams, D.; Järvelin, M.R.; Sebert, S. Vitamin D and the Promotion of Long-Term Metabolic Health from a Programming Perspective. Nutr. Metab. Insights 2016, 8, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [Green Version]
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 3, CD004905. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Available online: https://apps.who.int/iris/bitstream/handle/10665/250796/9789241549912-eng.pdf (accessed on 29 March 2022).
- Pereira-da-Silva, L.; Cabo, C.; Moreira, A.C.; Virella, D.; Guerra, T.; Camoes, T.; Silva, A.R.; Neves, R.; Ferreira, G.C. The adjusted effect of maternal body mass index, energy and macronutrient intakes during pregnancy, and gestational weight gain on body composition of full-term neonates. Am. J. Perinatol. 2014, 31, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hedderson, M.M.; Sridhar, S.; Xu, F.; Feng, J.; Ferrara, A. Poor diet quality in pregnancy is associated with increased risk of excess fetal growth: A prospective multi-racial/ethnic cohort study. Int. J. Epidemiol. 2019, 48, 423–432. [Google Scholar] [CrossRef]
- Brantsaeter, A.L.; Haugen, M.; Samuelsen, S.O.; Torjusen, H.; Trogstad, L.; Alexander, J.; Magnus, P.; Meltzer, H.M. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J. Nutr. 2009, 139, 1162–1168. [Google Scholar] [CrossRef] [Green Version]
- Larqué, E.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Koletzko, B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br. J. Nutr. 2012, 107, S77–S84. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.K.; Valentine, C.J.; Keim, S.A. DHA supplementation: Current implications in pregnancy and childhood. Pharmacol. Res. 2013, 70, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11, CD003402. [Google Scholar] [CrossRef]
- Izadi, V.; Tehrani, H.; Haghighatdoost, F.; Dehghan, A.; Surkan, P.J.; Azadbakht, L. Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition 2016, 32, 1092–1096. [Google Scholar] [CrossRef]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. Systematic review and meta-analysis of energy and macronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2012, 70, 322–336. [Google Scholar] [CrossRef]
- Stråvik, M.; Jonsson, K.; Hartvigsson, O.; Sandin, A.; Wold, A.E.; Sandberg, A.S.; Barman, M. Food and Nutrient Intake during Pregnancy in Relation to Maternal Characteristics: Results from the NICE Birth Cohort in Northern Sweden. Nutrients 2019, 11, 1680. [Google Scholar] [CrossRef] [Green Version]
- Jardí, C.; Aparicio, E.; Bedmar, C.; Aranda, N.; Abajo, S.; March, G.; Basora, J.; Arija, V.; The ECLIPSES Study Group. Food Consumption during Pregnancy and Post-Partum. ECLIPSES Study. Nutrients 2019, 11, 2447. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.C.; Zhou, S.J.; Flynn, A.C.; Malek, L.; Greco, R.; Moran, L. The Assessment of Diet Quality and Its Effects on Health Outcomes Pre-pregnancy and during Pregnancy. Semin. Reprod. Med. 2016, 34, 83–92. [Google Scholar] [CrossRef]
- Beslay, M.; Srour, B.; Méjean, C.; Allès, B.; Fiolet, T.; Debras, C.; Chazelas, E.; Deschasaux, M.; Wendeu-Foyet, M.G.; Hercberg, S.; et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: A prospective analysis of the French NutriNet-Santé cohort. PLoS Med 2020, 17, e1003256. [Google Scholar] [CrossRef]
- Parker, H.W.; Tovar, A.; McCurdy, K.; Vadiveloo, M. Associations between pre-pregnancy BMI, gestational weight gain, and prenatal diet quality in a national sample. PLoS ONE 2019, 14, e0224034. [Google Scholar] [CrossRef]
- World Health Organization. WHO Antenatal Care Recommendations for a Positive Pregnancy Experience—Nutritional Interventions Update: Multiple Micronutrient Supplements during Pregnancy. Available online: https://apps.who.int/iris/rest/bitstreams/1289394/retrieve (accessed on 29 March 2022).
- Hanson, M.A.; McAuliffe, F.M.; Killeen, S.L.; Jacob, C.M.; Hod, M. New guidelines, position paper, and insights from the FIGO Pregnancy Obesity and Nutrition Initiative (PONI). Int. J. Gynaecol. Obstet. 2020, 151, 1–3. [Google Scholar] [CrossRef]
- Massari, M.; Novielli, C.; Mandò, C.; Di Francesco, S.; Della Porta, M.; Cazzola, R.; Panteghini, M.; Savasi, V.; Maggini, S.; Schaefer, E.; et al. Multiple Micronutrients and Docosahexaenoic Acid Supplementation during Pregnancy: A Randomized Controlled Study. Nutrients 2020, 12, 2432. [Google Scholar] [CrossRef]
- SINU (Società Italiana di Nutrizione Umana—Italian Society of Human Nutrition). LARN. Nutrients and Energy Reference Intake Levels for Italian Population. Available online: https://eng.sinu.it/larn/ (accessed on 29 March 2022).
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Willett, W.C.; Sampson, L.; Stampfer, M.J.; Rosner, B.; Bain, C.; Witschi, J.; Hennekens, C.H.; Speizer, F.E. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985, 122, 51–65. [Google Scholar] [CrossRef]
- Vioque, J.; Navarrete-Muñoz, E.M.; Gimenez-Monzó, D.; García-de-la-Hera, M.; Granado, F.; Young, I.S.; Ramón, R.; Ballester, F.; Murcia, M.; Rebagliato, M.; et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr. J. 2013, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- BDA Working Group. Food Composition Database for Epidemiological Studies in Italy. Available online: www.bda-ieo.it (accessed on 29 March 2022).
- Rodríguez-Rodríguez, E.; Aparicio, A.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Serra-Majem, L.; Varela-Moreiras, G.; Ortega, R.M. Low Adherence to Dietary Guidelines in Spain, Especially in the Overweight/Obese Population: The ANIBES Study. J. Am. Coll. Nutr. 2017, 36, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Rogozińska, E.; Zamora, J.; Marlin, N.; Betrán, A.P.; Astrup, A.; Bogaerts, A.; Cecatti, J.G.; Dodd, J.M.; Facchinetti, F.; Geiker, N.R.W.; et al. Gestational weight gain outside the Institute of Medicine recommendations and adverse pregnancy outcomes: Analysis using individual participant data from randomised trials. BMC Pregnancy Childbirth. 2019, 19, 322. [Google Scholar] [CrossRef]
- Harrison, C.L.; Teede, H.; Khan, N.; Lim, S.; Chauhan, A.; Drakeley, S.; Moran, L.; Boyle, J. Weight management across preconception, pregnancy, and postpartum: A systematic review and quality appraisal of international clinical practice guidelines. Obes. Rev. 2021, 22, e13310. [Google Scholar] [CrossRef]
- Rugină, C.; Mărginean, C.O.; Meliţ, L.E.; Giga, D.V.; Modi, V.; Mărginean, C. Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women. J. Int. Med. Res. 2020, 48, 300060520933808. [Google Scholar] [CrossRef] [PubMed]
- Concina, F.; Pani, P.; Carletti, C.; Rosolen, V.; Knowles, A.; Parpinel, M.; Ronfani, L.; Mariuz, M.; Vecchi Brumatti, L.; Valent, F.; et al. Nutrient Intake during Pregnancy and Adherence to Dietary Recommendations: The Mediterranean PHIME Cohort. Nutrients 2021, 13, 1434. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, E.; Jardí, C.; Bedmar, C.; Pallejà, M.; Basora, J.; Arija, V.; The Eclipses Study Group. Nutrient Intake during Pregnancy and Post-Partum: ECLIPSES Study. Nutrients 2020, 12, 1325. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Lemieux, S.; Weisnagel, S.J.; Fontaine-Bisson, B.; Gagnon, C.; Robitaille, J.; Morisset, A.S. Trimester-Specific Dietary Intakes in a Sample of French-Canadian Pregnant Women in Comparison with National Nutritional Guidelines. Nutrients 2018, 10, 768. [Google Scholar] [CrossRef] [Green Version]
- Abeysekera, M.V.; Morris, J.A.; Davis, G.K.; O’Sullivan, A.J. Alterations in energy homeostasis to favour adipose tissue gain: A longitudinal study in healthy pregnant women. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 42–48. [Google Scholar] [CrossRef]
- Talai Rad, N.; Ritterath, C.; Siegmund, T.; Wascher, C.; Siebert, G.; Henrich, W.; Buhling, K.J. Longitudinal analysis of changes in energy intake and macronutrient composition during pregnancy and 6 weeks post-partum. Arch. Gynecol. Obstet. 2011, 283, 185–190. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14, 21–28. [Google Scholar] [CrossRef]
- Straßburg, A.; Eisinger-Watzl, M.; Krems, C.; Roth, A.; Hoffmann, I. Comparison of food consumption and nutrient intake assessed with three dietary assessment methods: Results of the German National Nutrition Survey II. Eur. J. Nutr. 2019, 58, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Román-Gálvez, M.R.; Amezcua-Prieto, C.; Salcedo-Bellido, I.; Olmedo-Requena, R.; Martínez-Galiano, J.M.; Khan, K.S.; Bueno-Cavanillas, A. Physical activity before and during pregnancy: A cohort study. Int. J. Gynaecol. Obstet. 2021, 152, 374–381. [Google Scholar] [CrossRef]
- Mulè, S.; Falla, M.; Conti, A.; Castiglione, D.; Blanco, I.; Platania, A.; D’Urso, M.; Marranzano, M. Macronutrient and Major Food Group Intake in a Cohort of Southern Italian Adults. Antioxidants 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein—Its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108, S105–S112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Armstrong, C.L.; Campbell, W.W. Effects of Dietary Protein Source and Quantity during Weight Loss on Appetite, Energy Expenditure, and Cardio-Metabolic Responses. Nutrients 2016, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Zerfu, T.A.; Mekuria, A. Pregnant women have inadequate fiber intake while consuming fiber-rich diets in low-income rural setting: Evidences from Analysis of common “ready-to-eat” stable foods. Food Sci. Nutr. 2019, 7, 3286–3292. [Google Scholar] [CrossRef]
- Rodríguez-Bernal, C.L.; Ramón, R.; Quiles, J.; Murcia, M.; Navarrete-Muñoz, E.M.; Vioque, J.; Ballester, F.; Rebagliato, M. Dietary intake in pregnant women in a Spanish Mediterranean area: As good as it is supposed to be? Public Health Nutr. 2013, 16, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Irles Rocamora, J.A.; Iglesias Bravo, E.M.; Avilés Mejías, S.; Bernal López, E.; de Valle Galindo, P.B.; Moriones López, L.; Maetzu Aznar, A.; Mingo Canal, D. Valor nutricional de la dieta en embarazadas sanas. Resultados de una encuesta dietética en gestantes. Nutr. Hosp. 2003, 18, 248–252. [Google Scholar]
- Damen, N.A.; Gillingham, M.; Hansen, J.G.; Thornburg, K.L.; Purnell, J.Q.; Marshall, N.E. Maternal dietary fat intake during pregnancy and newborn body composition. J. Perinatol. 2021, 41, 1007–1013. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [Green Version]
- EFSA Scientific Committee. Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. EFSA J. 2015, 13, 3982. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Cetin, I.; Brenna, J.T.; Alvino, G.; von Berlepsch, J.; Biesalski, H.K.; Clandini, T.; Debertin, H.; Decsi, T.; Demmelmair, H.; et al. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, E.C.; Geraghty, A.A.; Kilbane, M.T.; McKenna, M.J.; McAuliffe, F.M. Bone resorption and dietary calcium in pregnancy-a window to future maternal bone health. Osteoporos. Int. 2021, 32, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Gibbons, L.; Belizán, J.M. Impact of water fortification with calcium on calcium intake in different countries: A simulation study. Public Health Nutr. 2022, 25, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Galiano, J.M.; Amezcua-Prieto, C.; Cano-Ibañez, N.; Salcedo-Bellido, I.; Bueno-Cavanillas, A.; Delgado-Rodriguez, M. Maternal iron intake during pregnancy and the risk of small for gestational age. Matern. Child Nutr. 2019, 15, e12814. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Rawal, S. Dietary iron intake, iron status, and gestational diabetes. Am. J. Clin. Nutr. 2017, 106, 1672S–1680S. [Google Scholar] [CrossRef] [Green Version]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C.; INRAN-SCAI 2005–06 Study Group. The third Italian National Food Consumption Survey, INRAN–SCAI 2005–06—Part 1: Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [Green Version]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Parisi, F.; di Bartolo, I.; Savasi, V.M.; Cetin, I. Micronutrient supplementation in pregnancy: Who, what and how much? Obstet. Med. 2019, 12, 5–13. [Google Scholar] [CrossRef]
- Parisi, F.; Laoreti, A.; Cetin, I. Multiple micronutrient needs in pregnancy in industrialized countries. Ann. Nutr. Metab. 2014, 65, 13–21. [Google Scholar] [CrossRef]
- Caut, C.; Leach, M.; Steel, A. Dietary guideline adherence during preconception and pregnancy: A systematic review. Matern. Child Nutr. 2020, 16, e12916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Wang, Z.; Wang, L.; Wang, H.; Zhang, J.; Du, W.; Su, C.; Jia, X.; Ouyang, Y.; Wang, Y.; et al. Evaluating adherence to recommended diets in adults 1991–2015: Revised China dietary guidelines index. Nutr. J. 2019, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.J.; Guthrie, H.A.; Smiciklas-Wright, H. Accuracy of food portion estimation by overweight and normal-weight subjects. J. Am. Diet. Assoc. 1989, 89, 962–964. [Google Scholar] [CrossRef]



| Female Population Aged 30–59 yo Mean Height 1.65 m | EIP I TRIM (kCal) | EIP II TRIM (kCal) | EIP III TRIM (kCal) |
|---|---|---|---|
| ECP(69) + EIF | ECP(266) + EIF | ECP(496) + EIF | |
| PAL 1.45 | 2012 | 2209 | 2439 |
| PAL 1.6 | 2213 | 2410 | 2640 |
| PAL 1.75 | 2414 | 2611 | 2841 |
| Characteristics | Normal Weight (NW) = 133 | Over Weight (OW) = 43 |
|---|---|---|
| Age (years) | 133 31.4 ± 4.58 (32) | 43 32.2 ± 5.00 (34) |
| Pre-gestational Weight (kg) | 133 57.0 ± 6.42 (57) | 43 74.1 ± 7.43 (75) |
| Height (m) | 133 1.65 ± 0.06 (1.65) | 43 1.65 ± 0.06 (1.65) |
| Pre-gestational body mass index (BMI) (kg/m2) | 133 20.9 ± 1.91 (20.8) | 43 27.3 ± 1.54 (26.6) |
| Gestational Weight Gain (GWG) (kg) | 96 13.5 ± 4.59 (13) | 28 14.7 ± 7.09 (13.5) |
| Gestational Length (weeks) | 99 39.9 ± 1.07 (40) | 32 39.9 ± 1.41 (40) |
| Energy, Macro- and Micronutrients | I Trimester | II Trimester | III Trimester | |||
|---|---|---|---|---|---|---|
| NW (Mean ± st.dev) | OW (Mean ± st.dev) | NW (Mean ± st.dev) | OW (Mean ± st.dev) | NW (Mean ± st.dev) | OW (Mean ± st.dev) | |
| Calories, kcal | 2068.7 ± 759 | 1927.7 ± 733 | 1993.3 ± 761 | 1732.4 ± 614 | 2019 ± 783 | 1622.1 ± 623 * |
| Alcohol, g | 0.7 ± 2 | 0.3 ± 1 | 0.9 ± 2 | 0.6 ± 1 | 1 ± 3 | 0.8 ± 2 |
| Proteins, g | 81.3 ± 33 | 73.6 ± 24 | 78 ± 29 | 69.1 ± 21 | 81.2 ± 35 | 66.8 ± 29 * |
| Animal proteins, g | 49.8 ± 25 | 45.7 ± 18 | 48.7 ± 22 | 44.8 ± 16 | 50.6 ± 24 | 44.8 ± 20 |
| Vegetable proteins, g | 29.9 ± 15 | 26.4 ± 10 | 27.8 ± 11 | 23.1 ± 9 * | 28.9 ± 13 | 20.9 ± 10 * |
| Lipids, g | 68.9 ± 29 | 61.3 ± 22 | 66.2 ± 27 | 59 ± 21 | 63.5 ± 29 | 51.2 ± 20 * |
| Carbohydrates, g | 284.7 ± 115 | 275.5 ± 132 | 274.8 ± 121 | 235 ± 102 * | 283.4 ± 118 | 227.2 ± 104 * |
| Sugars, g | 112.6 ± 54 | 112.8 ± 65 | 114.7 ± 62 | 100.2 ± 55 | 115.8 ± 57 | 103 ± 64 |
| Dietary Fiber, g | 24.6 ± 12 | 22.6 ± 10 | 23.1 ± 10 | 19.3 ± 8 * | 24.2 ± 12 | 17.7 ± 10 * |
| Drinking water, mL | 1840.2 ± 737 | 1747.3 ± 595 | 1821.1 ± 695 | 1916.7 ± 543 | 1822 ± 700 | 1836.1 ± 702 |
| Cholesterol, mg | 260.6 ± 124 | 233.3 ± 96 | 251.1 ± 106 | 225.1 ± 79 | 261.6 ± 125 | 219.1 ± 104 * |
| Saturated fatty acids, g | 21.8 ± 9 | 19.5 ± 7 | 21 ± 9 | 18.9 ± 7 | 20.5 ± 10 | 16.4 ± 7 * |
| Polyunsaturated fatty acids, g | 10 ± 5 | 8.6 ± 3 | 9.7 ± 5 | 8.2 ± 3 | 9.3 ± 5 | 7.8 ± 4 * |
| Monounsaturated fatty acids, g | 29.3 ± 14 | 26.2 ± 11 | 27.6 ± 13 | 24.8 ± 10 | 26 ± 14 | 20.5 ± 9 * |
| C20_5 EPA, g | 0.287 ± 0 | 0.205 ± 0 | 0.275 ± 0 | 0.216 ± 0 | 0.296 ± 0 | 0.253 ± 0 |
| C22_6 DHA, g | 0.44 ± 0 | 0.318 ± 0 | 0.428 ± 0 | 0.343 ± 0 | 0.447 ± 0 | 0.402 ± 0 |
| Calcium, mg | 798 ± 413 | 685.5 ± 278 | 769.5 ± 332 | 674.4 ± 292 | 787.8 ± 390 | 688.5 ± 352 |
| Sodium, mg | 2892.8 ± 1871 | 2677.3 ± 2015 | 2662.8 ± 1716 | 2259.8 ± 1516 | 2535.7 ± 1681 | 2029.5 ± 1174 |
| Potassium, mg | 3705.4 ± 1614 | 3318.9 ± 1173 | 3680.9 ± 1395 | 3153.9 ± 1092 * | 3827.6 ± 1676 | 3125.6 ± 1712 * |
| Phosphorus, mg | 1292.6 ± 553 | 1140.9 ± 352 | 1248.6 ± 455 | 1086.3 ± 360 * | 1297.4 ± 548 | 1089.5 ± 493 * |
| Iron, mg | 12.5 ± 8 | 10.7 ± 4 | 11.8 ± 5 | 10.1 ± 4 * | 12.5 ± 6 | 10.2 ± 6 * |
| Zinc, mg | 13.2 ± 11 | 11.2 ± 5 | 12.2 ± 6 | 10.3 ± 5 | 13.3 ± 9 | 10.7 ± 6 |
| Folic acid, µg | 440.8 ± 223 | 400.1 ± 187 | 427.4 ± 234 | 345.3 ± 180 * | 455.5 ± 289 | 327.4 ± 217 * |
| Energy, Macro and Micronutrients | BMI | I Trimester | II Trimester | III Trimester |
|---|---|---|---|---|
| Calories, kcal | LARN | 2213 | 2410 | 2640 |
| NW | 2068.7 | 1993.3 | 2019.0 | |
| OW | 1927.7 | 1732.4 | 1622.1 | |
| Alcohol, g | LARN | 0 | 0 | 0 |
| NW | 0.7 | 0.9 | 1.0 | |
| OW | 0.3 | 0.6 | 0.8 | |
| Proteins, g | LARN | 55 | 62 | 80 |
| NW | 81.3 | 78.0 | 81.2 | |
| OW | 73.6 | 69.1 | 66.8 | |
| Proteins, % | LARN | 15 | 15 | 15 |
| NW | 15.7 | 15.6 | 16.1 | |
| OW | 15.3 | 15.9 | 16.5 | |
| Animal proteins, % | LARN | 67 | 67 | 67 |
| NW | 61.3 | 62.4 | 62.3 | |
| OW | 62.0 | 64.8 | 67.0 | |
| Vegetable proteins, % | LARN | 33 | 33 | 33 |
| NW | 36.8 | 35.6 | 35.6 | |
| OW | 35.9 | 33.5 | 31.2 | |
| Lipids, % | LARN | 20–35 | 20–35 | 20–35 |
| NW | 30.0 | 29.9 | 28.3 | |
| OW | 28.6 | 30.7 | 28.4 | |
| Carbohydrates, % | LARN | 45–60 | 45–60 | 45–60 |
| NW | 51.6 | 51.7 | 52.6 | |
| OW | 53.6 | 50.9 | 52.5 | |
| Sugars, % | LARN | <15 | <15 | <15 |
| NW | 20.4 | 21.6 | 21.5 | |
| OW | 22.0 | 21.7 | 23.8 | |
| Dietary Fiber, g | LARN | 25 | 25 | 25 |
| NW | 24.6 | 23.1 | 24.2 | |
| OW | 22.6 | 19.3 | 17.7 | |
| Drinking water, mL | LARN | 2350 | 2350 | 2350 |
| NW | 1840.2 | 1821.1 | 1822.0 | |
| OW | 1747.3 | 1916.7 | 1836.1 | |
| Cholesterol, mg | LARN | <300 | <300 | <300 |
| NW | 260.6 | 251.1 | 261.6 | |
| OW | 233.3 | 225.1 | 219.1 | |
| Saturated Fatty acids, % | LARN | <10 | <10 | <10 |
| NW | 9.5 | 9.5 | 9.1 | |
| OW | 9.1 | 9.8 | 9.1 | |
| Polyunsaturated Fatty acids, % | LARN | 5–10 | 5–10 | 5–10 |
| NW | 4.4 | 4.4 | 4.1 | |
| OW | 4.0 | 4.3 | 4.3 | |
| Monounsaturated Fatty acids, % | LARN | 5–10 | 5–10 | 5–10 |
| NW | 12.7 | 12.5 | 11.6 | |
| OW | 12.2 | 12.9 | 11.4 | |
| EPA + DHA, mg | LARN | 350–450 | 350–450 | 350–450 |
| NW | 727 | 703 | 743 | |
| OW | 523 | 559 | 655 | |
| Calcium, mg | LARN | 1200 | 1200 | 1200 |
| NW | 798.0 | 769.5 | 787.8 | |
| OW | 685.5 | 674.4 | 688.5 | |
| Sodium, mg | LARN | 1500 | 1500 | 1500 |
| NW | 2892.8 | 2662.8 | 2535.7 | |
| OW | 2677.3 | 2259.8 | 2029.5 | |
| Potassium, mg | LARN | 3900 | 3900 | 3900 |
| NW | 3705.4 | 3680.9 | 3827.6 | |
| OW | 3318.9 | 3153.9 | 3125.6 | |
| Phosphorus, mg | LARN | 700 | 700 | 700 |
| NW | 1292.6 | 1248.6 | 1297.4 | |
| OW | 1140.9 | 1086.3 | 1089.5 | |
| Iron mg | LARN | 27 | 27 | 27 |
| NW | 12.5 | 11.8 | 12.5 | |
| OW | 10.7 | 10.1 | 10.2 | |
| Zinc, mg | LARN | 12 | 12 | 12 |
| NW | 13.2 | 12.2 | 13.3 | |
| OW | 11.2 | 10.3 | 10.7 | |
| Folic acid, µg | LARN | 600 | 600 | 600 |
| NW | 440.8 | 427.4 | 455.5 | |
| OW | 400.1 | 345.3 | 327.4 |
| Recommended Frequency | % Adherence to Recommendation I Trimester | % Adherence to Recommendation II Trimester | % Adherence to Recommendation III Trimester | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALL | NW | OW | ALL | NW | OW | ALL | NW | OW | ||
| Bread | 2–5 portions/day | 12 | 14 | 7 | 11 | 11 | 13 | 8 | 10 | 8 |
| Breakfast Cereals | ≤3 portions/week | 78 | 75 | 88 | 77 | 75 | 83 | 55 | 75 | 86 |
| Pasta/Rice | 1–2 portions/day | 36 | 36 | 36 | 31 | 30 | 33 | 25 | 32 | 17 |
| Sweet bakery, biscuits | ≤2 portions/week | 27 | 26 | 29 | 26 | 21 | 38 | 19 | 23 | 22 |
| Fruit | ≥2 ½ portions/day | 38 | 38 | 36 | 36 | 37 | 33 | 27 | 40 | 22 |
| Vegetables | ≥2 ½ portions/day | 62 | 62 | 64 | 61 | 61 | 60 | 44 | 58 | 31 |
| White meat | 1–3 portions/week | 43 | 44 | 43 | 45 | 45 | 48 | 31 | 41 | 47 |
| Red meat | 1–2 portions/week | 42 | 44 | 33 | 38 | 38 | 38 | 29 | 42 | 42 |
| Processed and preserved meat | ≤1 portion/week | 31 | 27 | 43 | 37 | 35 | 45 | 22 | 39 | 33 |
| Fish | 2–3 portions/week | 18 | 17 | 19 | 23 | 23 | 25 | 12 | 26 | 25 |
| Preserved fish | ≤1 portion/week | 55 | 55 | 55 | 54 | 53 | 55 | 39 | 60 | 61 |
| Eggs | 2–4 portions/week | 28 | 30 | 21 | 28 | 30 | 23 | 20 | 28 | 19 |
| Legumes | 2–3 portions/week | 26 | 29 | 19 | 23 | 21 | 30 | 19 | 25 | 17 |
| Milk and Yogurt | 2–3 portions/day | 5 | 5 | 5 | 8 | 10 | 3 | 4 | 9 | 6 |
| Cheese and Aged Cheese | 2–3 portions/week | 18 | 15 | 26 | 17 | 16 | 18 | 12 | 17 | 31 |
| Oil | 2–4 portions/day | 29 | 29 | 31 | 26 | 25 | 30 | 21 | 25 | 11 |
| Nuts | ≤3 portions/week | 66 | 65 | 71 | 69 | 66 | 83 | 47 | 65 | 86 |
| Ultra-processed food | ≤1 portion/month | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Alcohols | 0 | 71 | 70 | 76 | 67 | 66 | 68 | 50 | 71 | 69 |
| Sugar, Honey, jam | ≤2 portions/day | 85 | 89 | 74 | 87 | 89 | 80 | 60 | 90 | 81 |
| Water | ≥8 portions/day | 18 | 20 | 14 | 22 | 22 | 23 | 13 | 19 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisso, F.; Massari, M.; Gentilucci, M.; Novielli, C.; Corti, S.; Nelva Stellio, L.; Milazzo, R.; Troiano, E.; Schaefer, E.; Cetin, I.; et al. Longitudinal Nutritional Intakes in Italian Pregnant Women in Comparison with National Nutritional Guidelines. Nutrients 2022, 14, 1944. https://doi.org/10.3390/nu14091944
Lisso F, Massari M, Gentilucci M, Novielli C, Corti S, Nelva Stellio L, Milazzo R, Troiano E, Schaefer E, Cetin I, et al. Longitudinal Nutritional Intakes in Italian Pregnant Women in Comparison with National Nutritional Guidelines. Nutrients. 2022; 14(9):1944. https://doi.org/10.3390/nu14091944
Chicago/Turabian StyleLisso, Fabrizia, Maddalena Massari, Micaela Gentilucci, Chiara Novielli, Silvia Corti, Leonardo Nelva Stellio, Roberta Milazzo, Ersilia Troiano, Ella Schaefer, Irene Cetin, and et al. 2022. "Longitudinal Nutritional Intakes in Italian Pregnant Women in Comparison with National Nutritional Guidelines" Nutrients 14, no. 9: 1944. https://doi.org/10.3390/nu14091944
APA StyleLisso, F., Massari, M., Gentilucci, M., Novielli, C., Corti, S., Nelva Stellio, L., Milazzo, R., Troiano, E., Schaefer, E., Cetin, I., & Mandò, C. (2022). Longitudinal Nutritional Intakes in Italian Pregnant Women in Comparison with National Nutritional Guidelines. Nutrients, 14(9), 1944. https://doi.org/10.3390/nu14091944