Alcohol, Drinking Pattern, and Chronic Disease
Abstract
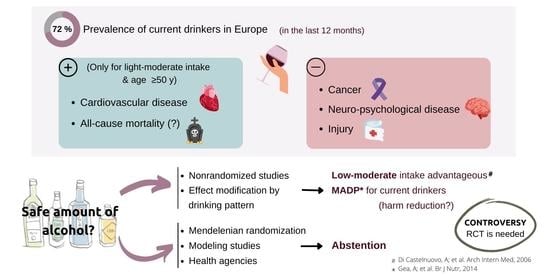
:1. Introduction
2. Methodological Problems in Nonrandomized Studies
3. Diverging Recommendations
4. Existing Randomized Controlled Trials in the Field of Alcohol Intervention
5. Mediterranean Alcohol Drinking Pattern, Wine in Moderation, and Reduced All-Cause Mortality
6. Mediterranean Diet and Mediterranean-Alcohol Drinking Pattern, A Complex Relationship
7. Alcohol Consumption and Chronic Diseases
7.1. Alcohol and CVD
7.2. Alcohol and Diabetes
7.3. Alcohol and Digestive Diseases
7.4. Alcohol and Cancer
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Sustainable Development Goals. Goal 3 Targets. Available online: https://www.un.org/sustainabledevelopment/health/ (accessed on 30 March 2022).
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef] [Green Version]
- Casswell, S. Will alcohol harm get the global response it deserves? Lancet 2019, 394, 1396–1397. [Google Scholar] [CrossRef]
- WHO. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- WHO Regional Office for Europe. Status Report on Alcohol Consumption, Harm and Policy Responses in 30 European Countries 2019; WHO Regional Office for Europe: Copenhagen, Denmark, 2019. [Google Scholar]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, P.I.; Islami, F.; Lemmens, P.V.E.P.P.; Rehm, P.J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Burton, R.; Sheron, N. No level of alcohol consumption improves health. Lancet 2018, 392, 987–988. [Google Scholar] [CrossRef] [Green Version]
- Goiana-Da-Silva, F.; Cruz-E-Silva, D.; Lindeman, M.; Hellman, M.; Angus, C.; Karlsson, T.; Renström, M.; Ferreira-Borges, C. Implementing the European Action Plan on Alcohol. Lancet Public Health 2019, 4, e493. [Google Scholar] [CrossRef] [Green Version]
- Rehm, J.; Baliunas, D.; Borges, G.L.G.; Graham, K.; Irving, H.; Kehoe, T.; Parry, C.D.; Patra, J.; Popova, S.; Poznyak, V.; et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction 2010, 105, 817–843. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, T.; Naimi, T. Study raises new doubts regarding the hypothesised health benefits of ‘moderate’ alcohol use. Evid. Based Med. 2016, 21, 156. [Google Scholar] [CrossRef]
- Naimi, T.S. Comment on Rehm: Alcohol, cohort studies and all-cause mortality: Where to from here? Drug Alcohol Rev. 2019, 38, 9–10. [Google Scholar] [CrossRef] [Green Version]
- Mukamal, K.J.; Conigrave, K.M.; Mittleman, M.A.; Camargo, C.A., Jr.; Stampfer, M.J.; Willett, W.C.; Rimm, E.B. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N. Engl. J. Med. 2003, 348, 109–118. [Google Scholar] [CrossRef]
- Ruidavets, J.-B.; Ducimetière, P.; Evans, A.; Montaye, M.; Haas, B.; Bingham, A.; Yarnell, J.; Amouyel, P.; Arveiler, D.; Kee, F.; et al. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: The Prospective Epidemiological Study of Myocardial Infarction (PRIME). BMJ 2010, 341, c6077. [Google Scholar] [CrossRef] [Green Version]
- Gea, A.; Bes-Rastrollo, M.; Toledo, E.; Garcia-Lopez, M.; Beunza, J.J.; Estruch, R.; Martinez-Gonzalez, M.A. Mediterranean alcohol-drinking pattern and mortality in the SUN (Seguimiento Universidad de Navarra) Project: A prospective cohort study. Br. J. Nutr. 2014, 111, 1871–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roerecke, M.; Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: A narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014, 12, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Hernandez, A.; Gea, A.; Ruiz-Canela, M.; Toledo, E.; Beunza, J.J.; Bes-Rastrollo, M.; Martinez-Gonzalez, M.A. Mediterranean Alcohol-Drinking Pattern and the Incidence of Cardiovascular Disease and Cardiovascular Mortality: The SUN Project. Nutrients 2015, 7, 9116–9126. [Google Scholar] [CrossRef] [Green Version]
- Bazal, P.; Gea, A.; Martínez-González, M.A.; Salas-Salvadó, J.; Asensio, E.M.; Muñoz-Bravo, C.; Fiol, M.; Muñoz, M.-A.; Lapetra, J.; Serra-Majem, L.L.; et al. Mediterranean alcohol-drinking pattern, low to moderate alcohol intake and risk of atrial fibrillation in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 676–683. [Google Scholar] [CrossRef]
- Rehm, J.; Hasan, O.S.M. Is burden of disease differentially linked to spirits? A systematic scoping review and implications for alcohol policy. Alcohol 2020, 82, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Martínez-González, M.A.; Barbería-Latasa, M.; Bes-Rastrollo, M.; Gea, A. Mediterranean diet, alcohol-drinking pattern and their combined effect on all-cause mortality: The Seguimiento Universidad de Navarra (SUN) cohort. Eur. J. Nutr. 2020, 60, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Faliva, M.A.; Gerbi, V.; La Vecchia, C.; Negri, E.; Opizzi, A.; Perna, S.; Pezzotti, M.; et al. Mediterranean Way of Drinking and Longevity. Crit. Rev. Food Sci. Nutr. 2016, 56, 635–640. [Google Scholar] [CrossRef]
- Boban, M.; Stockley, C.; Teissedre, P.-L.; Restani, P.; Fradera, U.; Stein-Hammer, C.; Ruf, J.-C. Drinking pattern of wine and effects on human health: Why should we drink moderately and with meals? Food Funct. 2016, 7, 2937–2942. [Google Scholar] [CrossRef] [Green Version]
- Bell, S.; Daskalopoulou, M.; Rapsomaniki, E.; George, J.; Britton, A.; Bobak, M.; Casas, J.P.; Dale, C.E.; Denaxas, S.; Shah, A.D.; et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: Population based cohort study using linked health records. BMJ 2017, 356, j909. [Google Scholar] [CrossRef] [Green Version]
- Ronksley, P.E.; Brien, S.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ 2011, 342, d671. [Google Scholar] [CrossRef] [Green Version]
- Corrao, G.; Bagnardi, V.; Zambon, A.; La Vecchia, C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev. Med. 2004, 38, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; Costanzo, S.; Bagnardi, V.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Alcohol dosing and total mortality in men and women: An updated meta-analysis of 34 prospective studies. Arch. Intern. Med. 2006, 166, 2437–2445. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Jung, J.-G.; Lee, S.; Kim, J.-S.; Ahn, S.-K.; Shin, E.-S.; Jang, J.-E.; Lim, S.-H. The protective effect of alcohol consumption on the incidence of cardiovascular diseases: Is it real? A systematic review and meta-analysis of studies conducted in community settings. BMC Public Health 2020, 20, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britton, K.A.; Gaziano, J.M.; Sesso, H.D.; Djousse, L. Relation of alcohol consumption and coronary heart disease in hypertensive male physicians (from the Physicians’ Health Study). Am. J. Cardiol. 2009, 104, 932–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukamal, K.J.; Chiuve, S.E.; Rimm, E.B. Alcohol consumption and risk for coronary heart disease in men with healthy lifestyles. Arch. Intern. Med. 2006, 166, 2145–2150. [Google Scholar] [CrossRef] [Green Version]
- Hvidtfeldt, U.A.; Tolstrup, J.S.; Jakobsen, M.U.; Heitmann, B.L.; Grønbæk, M.; O’Reilly, E.; Bälter, K.; Goldbourt, U.; Hallmans, G.; Knekt, P.; et al. Alcohol intake and risk of coronary heart disease in younger, middle-aged, and older adults. Circulation 2010, 121, 1589–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arriola, L.; Camblor, P.M.; Larrañaga, N.; Basterretxea, M.; Amiano, P.; Moreno-Iribas, C.; Carracedo, R.; Agudo, A.; Ardanaz, E.; Barricarte, A.; et al. Alcohol intake and the risk of coronary heart disease in the Spanish EPIC cohort study. Heart 2010, 96, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Howard, A.A.; Arnsten, J.H.; Gourevitch, M.N. Effect of alcohol consumption on diabetes mellitus: A systematic review. Ann. Intern. Med. 2004, 140, 211. [Google Scholar] [CrossRef]
- Holman, C.D.; English, D.R.; Milne, E.; Winter, M.G. Meta-analysis of alcohol and all-cause mortality: A validation of NHMRC recommendations. Med. J. Aust. 1996, 164, 141–145. [Google Scholar] [CrossRef]
- Gmel, G.; Gutjahr, E.; Rehm, J. How stable is the risk curve between alcohol and all-cause mortality and what factors influence the shape? A precision-weighted hierarchical meta-analysis. Eur. J. Epidemiol. 2003, 18, 631–642. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality in relation to alcohol consumption: A prospective study among male British doctors. Int. J. Epidemiol. 2005, 34, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellavia, A.; Bottai, M.; Wolk, A.; Orsini, N. Alcohol consumption and mortality: A dose-response analysis in terms of time. Ann. Epidemiol. 2014, 24, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, E.; Mukamal, K.J.; Giovannucci, E.L.; Stampfer, M.J.; Rimm, E.B. Key findings on alcohol consumption and a variety of health outcomes from the Nurses’ Health Study. Am. J. Public Health 2016, 106, 1586–1591. [Google Scholar] [CrossRef]
- Li, Y.; Pan, A.; Wang, D.D.; Liu, X.; Dhana, K.; Franco, O.H.; Kaptoge, S.; Di Angelantonio, E.; Stampfer, M.; Willett, W.C.; et al. Impact of Healthy Lifestyle Factors on Life Expectancies in the US Population. Circulation 2018, 138, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Keyes, K.M.; Calvo, E.; Ornstein, K.A.; Rutherford, C.; Fox, M.P.; Staudinger, U.M.; Fried, L.P. Alcohol Consumption in Later Life and Mortality in the United States: Results from 9 Waves of the Health and Retirement Study. Alcohol Clin. Exp. Res. 2019, 43, 1734–1746. [Google Scholar] [CrossRef]
- Zhao, J.; Stockwell, T.; Roemer, A.; Naimi, T.; Chikritzhs, T. Alcohol Consumption and Mortality from Coronary Heart Disease: An Updated Meta-Analysis of Cohort Studies. J. Stud. Alcohol Drugs 2017, 78, 375–386. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Barbería-Latasa, M.; Pérez de Rojas, J.; Domínguez Rodriguez, L.J.; Gea, A. Alcohol and early mortality (before 65 years) in the ‘Seguimiento Universidad de Navarra’ (SUN) cohort: Does any level reduce mortality? Br. J. Nutr. 2021, 127, 1415–1425. [Google Scholar] [CrossRef]
- Rehm, J. Why the relationship between level of alcohol-use and all-cause mortality cannot be addressed with meta-analyses of cohort studies. Drug Alcohol Rev. 2019, 38, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, T. Greater precision at the price of greater uncertainty? A response to Rehm. Drug Alcohol Rev. 2019, 38, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Angus, C.; Holmes, J. The allure, and challenges, of complexity in epidemiological modelling of alcohol harm: Commentary on Rehm. Drug Alcohol Rev. 2019, 38, 11–12. [Google Scholar] [CrossRef] [Green Version]
- Naimi, T.S.; Stockwell, T.; Zhao, J.; Xuan, Z.; Dangardt, F.; Saitz, R.; Liang, W.; Chikritzhs, T. Selection biases in observational studies affect associations between ‘moderate’ alcohol consumption and mortality. Addiction 2017, 112, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, K.M.; Kerr, W.C.; Stockwell, T.; Chikritzhs, T.; Bostrom, A. Moderate alcohol use and reduced mortality risk: A systematic error in prospective studies. Addict Res. Theory 2006, 14, 101–132. [Google Scholar] [CrossRef]
- Naimi, T.S.; Brown, D.W.; Brewer, R.D.; Giles, W.H.; Mensah, G.; Serdula, M.K.; Mokdad, A.H.; Hungerford, D.W.; Lando, J.; Naimi, S.; et al. Cardiovascular risk factors and confounders among nondrinking and moderate-drinking US. Adults. Am. J. Prev. Med. 2005, 28, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Dale, C.E.; Zuccolo, L.; Silverwood, R.J.; Guo, Y.; Ye, Z.; Prieto-Merino, D.; Dehghan, A.; Trompet, S.; Wong, A.; et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 2014, 349, g4164. [Google Scholar] [CrossRef] [Green Version]
- Millwood, I.Y.; Walters, R.G.; Mei, X.W.; Guo, Y.; Yang, L.; Bian, Z.; Bennett, D.A.; Chen, Y.; Dong, C.; Hu, R.; et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: A prospective study of 500.000 men and women in China. Lancet 2019, 393, 1831–1842. [Google Scholar] [CrossRef] [Green Version]
- Chikritzhs, T.; Stockwell, T.; Naimi, T.; Andreasson, S.; Dangardt, F.; Liang, W. Has the leaning tower of presumed health benefits from “moderate” alcohol use finally collapsed? Addiction 2015, 110, 726–727. [Google Scholar] [CrossRef]
- Yang, J.H.; Jeong, J.A.; Kweon, S.S.; Lee, Y.H.; Choi, S.W.; Ryu, S.Y.; Nam, H.S.; Park, K.S.; Kim, H.Y.; Shin, M.H. Causal Association Between Alcohol Consumption and Atrial Fibrillation: A Mendelian Randomization Study. Korean Circ. J. 2022, 52, 220–230. [Google Scholar] [CrossRef]
- Rehm, J. Alcohol consumption and all-cause mortality: Further implications. Drug Alcohol Rev. 2019, 38, 13–15. [Google Scholar] [CrossRef] [Green Version]
- Swanson, S.A.; Tiemeier, H.; Ikram, M.A.; Hernan, M.A. Nature as a Trialist? Deconstructing the Analogy Between Mendelian Randomization and Randomized Trials. Epidemiology 2017, 28, 653–659. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Stampfer, M.J.; Rimm, E.B. Genetic instrumental variable analysis: Time to call mendelian randomization what it is. The example of alcohol and cardiovascular disease. Eur. J. Epidemiol. 2020, 35, 93–97. [Google Scholar] [CrossRef]
- GBD 2016 Alcohol and Drug Use Collaborators. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 2018, 5, 987–1012. [Google Scholar] [CrossRef] [Green Version]
- Mukamal, K.J. A safe level of alcohol consumption: The right answer demands the right question. J. Intern. Med. 2020, 288, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J.; Clowry, C.M.; Murray, M.M.; Hendriks, H.F.; Rimm, E.B.; Sink, K.M.; Adebamowo, C.A.; Dragsted, L.O.; Lapinski, P.S.; Lazo, M.; et al. Moderate Alcohol Consumption and Chronic Disease: The Case for a Long-Term Trial. Alcohol Clin. Exp. Res. 2016, 40, 2283–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psaltopoulou, T.; Sergentanis, T.N.; Ntanasis-Stathopoulos, I.; Tzanninis, I.G.; Tsilimigras, D.I.; Dimopoulos, M.A. Alcohol consumption and risk of hematological malignancies: A meta- analysis of prospective studies. Int. J. Cancer 2018, 143, 486–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imtiaz, S.; Shield, K.D.; Roerecke, M.; Samokhvalov, A.V.; Lonnroth, K.; Rehm, J. Alcohol consumption as a risk factor for tuberculosis: Meta-analyses and burden of disease. Eur. Respir. J. 2017, 50, 1700216. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.; Manthey, J.; Rylett, M.; Probst, C.; Wettlaufer, A.; Parry, C.D.H.; Rehm, J. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: A comparative risk assessment study. Lancet Public Health 2020, 5, e51–e61. [Google Scholar] [CrossRef] [Green Version]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef]
- Sutanto, H.; Cluitmans, M.J.M.; Dobrev, D.; Volders, P.G.A.; Bébarová, M.; Heijman, J. Acute effects of alcohol on cardiac electrophysiology and arrhythmogenesis: Insights from multiscale in silico analyses. J. Mol. Cell Cardiol. 2020, 146, 69–83. [Google Scholar] [CrossRef]
- Liu, R.; Sun, F.; Armand, L.C.; Wu, R.; Xu, C. Chronic Ethanol Exposure Induces Deleterious Changes in Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. Stem. Cell Rev. Rep. 2021, 17, 2314–2331. [Google Scholar] [CrossRef]
- Singh, S.; Chen, Y.; Matsumoto, A.; Orlicky, D.J.; Dong, H.; Thompson, D.C.; Vasiliou, V. ALDH1B1 links alcohol consumption and diabetes. Biochem. Biophys. Res. Commun. 2015, 463, 768–773. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Cao, Q.; Wang, C.; Ma, X.; Shen, C.; Liu, X.W.; Bu, L.P.; Zou, Y.Z.; Hu, K.; Sun, A.J.; et al. Impact of chronic low to moderate alcohol consumption on blood lipid and heart energy profile in acetaldehyde dehydrogenase 2-deficient mice. Acta Pharmacol. Sin. 2014, 35, 1015–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutanto, H.; Dobrev, D.; Heijman, J. Resveratrol: An effective pharmacological agent to prevent inflammation-induced atrial fibrillation? Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 1163–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Xu, M.; Li, F.; Wang, X.; Bower, K.A.; Frank, J.A.; Lu, Y.; Chen, G.; Zhang, Z.; Ke, Z.; et al. Ethanol promotes mammary tumor growth and angiogenesis: The involvement of chemoattractant factor MCP-1. Breast Cancer Res. Treat. 2012, 133, 1037–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Ren, Z.; Wang, X.; Comer, A.; Frank, J.A.; Ke, Z.J.; Huang, Y.; Zhang, Z.; Shi, X.; Wang, S.; et al. ErbB2 and p38γ MAPK mediate alcohol-induced increase in breast cancer stem cells and metastasis. Mol. Cancer 2016, 15, 52. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Wang, S.; Ren, Z.; Frank, J.A.; Yang, X.H.; Zhang, Z.; Ke, Z.J.; Shi, X.; Luo, J. Chronic ethanol exposure enhances the aggressiveness of breast cancer: The role of p38γ. Oncotarget 2016, 7, 3489–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Howard, E.W.; Parris, A.B.; Guo, Z.; Zhao, Q.; Yang, X. Alcohol promotes migration and invasion of triple-negative breast cancer cells through activation of p38 MAPK and JNK. Mol. Carcinog. 2017, 56, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.F.; Zhou, Y.; Adams, D.J.; Arends, M.J. Effects of long-term ethanol consumption and Aldh1b1 depletion on intestinal tumourigenesis in mice. J. Pathol. 2017, 241, 649–660. [Google Scholar] [CrossRef] [Green Version]
- Klein, W.M.P.; Jacobsen, P.B.; Helzlsouer, K.J. Alcohol and cancer risk. Clinical and research implications. JAMA 2020, 323, 23–24. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- McKenzie, F.; Biessy, C.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Chajès, V.; Dahm, C.; Overvad, K.; Dossus, L.; Lagiou, P.; et al. Healthy Lifestyle and Risk of Cancer in the European Prospective Investigation in to Cancer and Nutrition Cohort Study. Medicine 2016, 95, e2850. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J.; Giovannucci, E.L. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: Results from two prospective US cohort studies. BMJ 2015, 351, h4238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, R.F.; Hermon, C.; Liu, B.; Green, J.; Reeves, G.K.; Beral, V.; Floud, S. Million Women Study Collaborators. Alcohol drinking patterns and liver cirrhosis risk: Analysis of the prospective UK Million Women Study. Lancet Public Health 2019, 4, e41–e48. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Li, X.; Zhou, T.; Sun, D.; Shai, I.; Heianza, Y.; Rimm, E.B.; Manson, J.E.; Qi, L. Alcohol Consumption Levels as Compared With Drinking Habits in Predicting All-Cause Mortality and Cause-Specific Mortality in Current Drinkers. Mayo. Clin. Proc. 2021, 96, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Jani, B.D.; McQueenie, R.; Nicholl, B.I.; Field, R.; Hanlon, P.; Gallacher, K.I.; Mair, F.S.; Lewsey, J. Association between patterns of alcohol consumption (beverage type, frequency and consumption with food) and risk of adverse health outcomes: A prospective cohort study. BMC Med. 2021, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Sánchez-Villegas, A. The emerging role of Mediterranean diets in cardiovascular epidemiology: Monounsaturated fats olive oil red wine or the whole pattern? Eur. J. Epidemiol. 2004, 19, 9–13. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Klatsky, A.L. Alcohol and cardiovascular mortality: Common sense and scientific truth. J. Am. Coll. Cardiol. 2010, 55, 1336–1338. [Google Scholar] [CrossRef] [Green Version]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health: A Comprehensive Review. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef]
- Pavlidou, E.; Mantzorou, M.; Fasoulas, A.; Tryfonos, C.; Petridis, D.; Giaginis, C. Wine: An Aspiring Agent in Promoting Longevity and Preventing Chronic Diseases. Diseases 2018, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [Green Version]
- Shaper, A.G.; Wannamethee, G.; Walker, M. Alcohol and mortality in British men: Explaining the U-shaped curve. Lancet 1988, 2, 1267–1273. [Google Scholar] [CrossRef]
- Fillmore, K.M.; Stockwell, T.; Chikritzhs, T.; Bostrom, A.; Kerr, W. Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies and new hypotheses. Ann. Epidemiol. 2007, 17 (Suppl. S5), S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, T.; Zhao, J.; Panwar, S.; Roemer, A.; Naimi, T.; Chikritzhs, T. Do “moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J. Stud. Alcohol Drugs 2016, 77, 185–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockwell, T.; Zhao, J.; Macdonald, S. Who under-reports their alcohol consumption in telephone surveys and by how much? An application of the “yesterday method” in a national Canadian substance use survey. Addiction 2014, 109, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Esser, M.B.; Sherk, A.; Subbaraman, M.S.; Martinez, P.; Karriker-Jaffe, K.J.; Sacks, J.J.; Naimi, T.S. Improving Estimates of Alcohol-Attributable Deaths in the United States: Impact of Adjusting for the Underreporting of Alcohol Consumption. J. Stud. Alcohol Drugs 2022, 83, 134–144. [Google Scholar] [CrossRef]
- Vance, M.C.; Caverly, T.J.; Hayward, R.A. Underappreciated Bias Created by Measurement Error in Risk Factor Assessment-A Case Study of No Safe Level of Alcohol Consumption. JAMA Intern. Med. 2019, 180, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Sterling, S.A.; Palzes, V.A.; Lu, Y.; Kline-Simon, A.H.; Parthasarathy, S.; Ross, T.; Elson, J.; Weisner, C.; Maxim, C.; Chi, F.W. Associations Between Medical Conditions and Alcohol Consumption Levels in an Adult Primary Care Population. JAMA Netw. Open. 2020, 3, e204687. [Google Scholar] [CrossRef]
- Palzes, V.A.; Kline-Simon, A.H.; Satre, D.D.; Sterling, S.; Weisner, C.; Chi, F.W. Predictors of early and sustained cessation of heavy drinking over 5 years among adult primary care patients. Addiction 2022, 117, 82–95. [Google Scholar] [CrossRef]
- Davey Smith, G.; Holmes, M.V.; Davies, N.M.; Ebrahim, S. Mendel’s laws, Mendelian randomization and causal inference in observational data: Substantive and nomenclatural issues. Eur. J. Epidemiol. 2020, 35, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Forbes, S.P.; Dahabreh, I.J. Benchmarking Observational Analyses Against Randomized Trials: A Review of Studies Assessing Propensity Score Methods. J. Gen. Intern. Med. 2020, 35, 1396–1404. [Google Scholar] [CrossRef]
- Califf, R.M.; Hernandez, A.F.; Landray, M. Weighing the Benefits and Risks of Proliferating Observational Treatment Assessments: Observational Cacophony, Randomized Harmony. JAMA 2020, 324, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Bowman, L.; Landray, M.; Peto, R. The Magic of Randomization versus the Myth of Real-World Evidence. N. Engl. J. Med. 2020, 382, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, D.; Lovato, L.C.; Khudyakov, P.; Wilkens, T.L.; Adebamowo, C.A.; Adebamowo, S.N.; Appel, L.J.; Beulens, J.W.; Coughlin, J.W.; Dragsted, L.O.; et al. The Moderate Alcohol and Cardiovascular Health Trial (MACH15): Design and methods for a randomized trial of moderate alcohol consumption and cardiometabolic risk. Eur. J. Prev. Cardiol. 2020, 27, 1967–1982. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Anderson, C.A.; Ix, J.H. Editorial: From MACH15 to MACH0—A missed opportunity to understand the health effects of moderate alcohol intake. Eur. J. Prev. Cardiol. 2020, 28, e23–e24. [Google Scholar] [CrossRef] [Green Version]
- Brien, S.E.; Ronsksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: A systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrieks, I.C.; Heil, A.L.; Hendriks, H.F.; Mukamal, K.J.; Beulens, J.W. The effect of alcohol consumption on insulin sensitivity and glycemic status: A systematic review and meta-analysis of intervention studies. Diabetes Care 2015, 38, 723–732. [Google Scholar] [CrossRef]
- Deykin, D.; Janson, P.; McMahon, L. Ethanol potentiation of aspirin-induced prolongation of the bleeding time. N. Engl. J. Med. 1982, 306, 852–854. [Google Scholar] [CrossRef]
- Mahabir, S.; Baer, D.J.; Johnson, L.L.; Dorgan, J.F.; Campbell, W.; Brown, E.; Hartman, T.J.; Clevidence, B.; Albanes, D.; Judd, J.T.; et al. The effects of moderate alcohol supplementation on estrone sulfate and DHEAS in postmenopausal women in a controlled feeding study. Nutr. J. 2004, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Reichman, M.E.; Judd, J.T.; Longcope, C.; Schatzkin, A.; Clevidence, B.A.; Nair, P.; Campbell, W.S.; Taylor, P.R. Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J. Natl. Cancer Inst. 1993, 85, 722–727. [Google Scholar] [CrossRef]
- Hartman, T.J.; Baer, D.J.; Graham, L.B.; Stone, W.L.; Gunter, E.W.; Parker, C.E.; Albert, P.S.; Dorgan, J.F.; Clevidence, B.A.; Campbell, W.S.; et al. Moderate alcohol consumption and levels of antioxidant vitamins and isoprostanes in postmenopausal women. Eur. J. Clin. Nutr. 2005, 59, 161–168. [Google Scholar] [CrossRef]
- Assi, N.; Rinaldi, S.; Viallon, V.; Dashti, S.G.; Dossus, L.; Fournier, A.; Cervenka, I.; Kvaskoff, M.; Turzanski-Fortner, R.; Bergmann, M.; et al. Mediation analysis of the alcohol-postmenopausal breast cancer relationship by sex hormones in the EPIC cohort. Int. J. Cancer 2020, 146, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: A 2-year randomized, controlled trial. Ann. Intern. Med. 2015, 163, 569–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfella, R.; Cacciapuoti, F.; Siniscalchi, M.; Sasso, F.C.; Marchese, F.; Cinone, F.; Musacchio, E.; Marfella, M.A.; Ruggiero, L.; Chiorazzo, G.; et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with Type 2 diabetes mellitus. Diabet. Med. 2006, 23, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Rimm, E.B.; Williams, P.; Fosher, K.; Criqui, M.; Stampfer, M.J. Moderate alcohol intake and lower risk of coronary heart disease: Meta-analysis of effects on lipids and haemostatic factors. BMJ 1999, 319, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Puddey, I.B.; Beilin, L.J.; Vandongen, R.; Rouse, I.L.; Rogers, P. Evidence for a direct effect of alcohol consumption on blood pressure in normotensive men. A randomized controlled trial. Hypertension 1985, 7, 707–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shai, I.; Wainstein, J.; Harman-Boehm, I.; Raz, I.; Fraser, D.; Rudich, A.; Stampfer, M.J. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: A multicenter, randomized, clinical intervention trial. Diabetes Care 2007, 30, 3011–3016. [Google Scholar] [CrossRef] [Green Version]
- Sierksma, A.; van der Gaag, M.S.; Kluft, C.; Hendriks, H.F. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur. J. Clin. Nutr. 2002, 56, 1130–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierksma, A.; Sarkola, T.; Eriksson, C.J.; van der Gaag, M.S.; Grobbee, D.E.; Hendriks, H.F. Effect of moderate alcohol consumption on plasma dehydroepiandrosterone sulfate, testosterone, and estradiol levels in middle-aged men and postmenopausal women: A diet-controlled intervention study. Alcohol Clin. Exp. Res. 2004, 28, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, Y.; Zheng, S.; Yang, X.; Wang, T.; Zeng, J. Moderate alcohol consumption and atherosclerosis: Meta-analysis of effects on lipids and inflammation. Wien. Klin. Wochenschr. 2017, 129, 835–843. [Google Scholar] [CrossRef]
- Kaner, E.F.; Dickinson, H.O.; Beyer, F.R.; Campbell, F.; Schlesinger, C.; Heather, N.; Saunders, J.B.; Burnand, B.; Pienaar, E.D. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst. Rev. 2018, 2, CD004148. [Google Scholar] [CrossRef] [Green Version]
- Kaner, E.F.; Beyer, F.R.; Garnett, C.; Crane, D.; Brown, J.; Muirhead, C.; Redmore, J.; O’Donnell, A.; Newham, J.J.; de Vocht, F. Personalised digital interventions for reducing hazardous and harmful alcohol consumption in community-dwelling populations. Cochrane Database Syst. Rev. 2017, 9, CD011479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, M.; Puddephatt, J.A.; Goodwin, L.; Owens, L.; Reaves, D.; Holmes, J. Benefits of temporary alcohol restriction: A feasibility randomized trial. Pilot Feasibility Stud. 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grekin, E.R.; Beatty, J.R.; McGoron, L.; Kugler, K.C.; McClure, J.B.; Pop, D.E.; Ondersma, S.J. Testing the efficacy of motivational strategies, empathic reflections, and lifelike features in a computerized intervention for alcohol use: A factorial trial. Psychol. Addict. Behav. 2019, 33, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Dedert, E.A.; McDuffie, J.R.; Stein, R.; McNiel, J.M.; Kosinski, A.S.; Freiermuth, C.E.; Hemminger, A.; Williams, J.W., Jr. Electronic Interventions for Alcohol Misuse and Alcohol Use Disorders: A Systematic Review. Ann. Intern. Med. 2015, 163, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Jonas, D.E.; Garbutt, J.C.; Amick, H.R.; Brown, J.M.; Brownley, K.A.; Council, C.L.; Viera, A.J.; Wilkins, T.M.; Schwartz, C.J.; Richmond, E.M. Behavioral counseling after screening for alcohol misuse in primary care: A systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2012, 157, 645–654. [Google Scholar] [CrossRef]
- O’Connor, E.A.; Perdue, L.A.; Senger, C.A.; Rushkin, M.; Patnode, C.D.; Bean, S.I.; Jonas, D.E. Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 1910–1928. [Google Scholar] [CrossRef] [Green Version]
- Voskoboinik, A.; Kalman, J.M.; De Silva, A.; Nicholls, T.; Costello, B.; Nanayakkara, S.; Prabhu, S.; Stub, D.; Azzopardi, S.; Vizi, D.; et al. Alcohol abstinence in drinkers with atrial fibrillation. N. Engl. J. Med. 2020, 382, 20–28. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Criqui, M.H.; Rigel, B.L. Does diet or alcohol explain the French paradox? Lancet 1994, 344, 1719–1723. [Google Scholar] [CrossRef]
- Lippi, G.; Franchini, M.; Favaloro, E.J.; Targher, G. Moderate red wine consumption and cardiovascular disease risk: Beyond the “French paradox”. Semin. Thromb. Hemost. 2010, 36, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Fragopoulou, E.; Antonopoulou, S. The French paradox three decades later: Role of inflammation and thrombosis. Clin. Chim. Acta 2020, 510, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Grønbaek, M.; Becker, U.; Johansen, D.; Gottschau, A.; Schnohr, P.; Hein, H.O.; Jensen, G.; Sørensen, T.I. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 2000, 133, 411–419. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, G.; Cerletti, C. Wine and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2001, 11 (Suppl. S4), 47–50. [Google Scholar] [PubMed]
- Fernández-Jarne, E.; Martínez-Losa, E.; Serrano-Martínez, M.; Prado-Santamaría, M.; Brugarolas-Brufau, C.; Martínez-González, M.A. Type of alcoholic beverage and first acute myocardial infarction: A case-control study in a Mediterranean country. Clin. Cardiol. 2003, 26, 313–318. [Google Scholar] [CrossRef]
- Harriss, L.R.; English, D.R.; Hopper, J.L.; Powles, J.; Simpson, J.A.; O’Dea, K.; Giles, G.G.; Tonkin, A.M. Alcohol consumption and cardiovascular mortality accounting for possible misclassification of intake: 11-year follow-up of the Melbourne Collaborative Cohort Study. Addiction 2007, 102, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.T.; Ocké, M.C.; Boshuizen, H.C.; Kok, F.J.; Kromhout, D. Long-term wine consumption is related to cardiovascular mortality and life expectancy independently of moderate alcohol intake: The Zutphen Study. J. Epidemiol. Community Health 2009, 63, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 2009, 338, b2337. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. S6), 1402S–1406S. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Trichopoulos, D.; Lagiou, P. Dietary patterns and mortality. Br. J. Nutr. 2001, 85, 133–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. Dietary patterns and their association with food and nutrient intake in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Br. J. Nutr. 2001, 85, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grønbaek, M.; Tjønneland, A.; Johansen, D. Type of alcohol and drinking pattern in 56,970 Danish men and women. Eur. J. Clin. Nutr. 2000, 54, 174–176. [Google Scholar] [CrossRef] [Green Version]
- Maso, L.D.; La Vecchia, C.; Polesel, J.; Talamini, R.; Levi, F.; Conti, E.; Zambon, P.; Negri, E.; Franceschi, S. Alcohol drinking outside meals and cancers of the upper aero-digestive tract. Int. J. Cancer 2002, 102, 435–437. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar]
- Sergent, O.; Griffon, B.; Cillard, P.; Cillard, J. Alcool et stress oxydatif. Pathol. Biol. 2001, 49, 689–695. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Aung, S.; Nah, G.; Vittinghoff, E.; Groh, C.A.; Fang, C.D.; Marcus, G.M. Population-level analyses of alcohol consumption as a predictor of acute atrial fibrillation episodes. Nat. Cardiovasc Res. 2022, 1, 23–27. [Google Scholar] [CrossRef]
- Biddinger, K.J.; Emdin, C.A.; Haas, M.E.; Wang, M.; Hindy, G.; Ellinor, P.T.; Kathiresan, S.; Khera, A.V.; Aragam, K.G. Association of Habitual Alcohol Intake with Risk of Cardiovascular Disease. JAMA Netw. Open. 2022, 5, e223849. [Google Scholar] [CrossRef]
- Ortolá, R.; García-Esquinas, E.; Buño-Soto, A.; Carballo-Casla, A.; Sotos-Prieto, M.; Banegas, J.R.; Rodríguez-Artalejo, F. Alcohol consumption patterns and growth differentiation factor 15 among life-time drinkers aged 65+ years in Spain: A cross-sectional study. Addiction 2022, 117, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.T.; Karter, A.J.; Warton, E.M.; Doan, J.U.; Weisner, C.M. The relationship between alcohol consumption and glycemic control among patients with diabetes: The Kaiser Permanente Northern California Diabetes Registry. J. Gen. Intern. Med. 2008, 23, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirst, J.A.; Aronson, J.K.; Feakins, B.G.; Ma, C.; Farmer, A.J.; Stevens, R.J. Short- and medium-term effects of light to moderate alcohol intake on glycaemic control in diabetes mellitus: A systematic review and meta-analysis of randomized trials. Diabet. Med. 2017, 34, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.T.; Karter, A.J.; Liu, J. Alcohol consumption is inversely associated with adherence to diabetes self-care behaviours. Diabet. Med. 2006, 23, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, B.; Rehm, J.; Gmel, G. Moderate alcohol consumption and the gastrointestinal tract. Dig. Dis. 2005, 23, 170–176. [Google Scholar] [CrossRef]
- Rocco, A.; Compare, D.; Angrisani, D.; Sanduzzi Zamparelli, M.; Nardone, G. Alcoholic disease: Liver and beyond. World J. Gastroenterol. 2014, 20, 14652–14659. [Google Scholar] [CrossRef] [PubMed]
- Kamper-Jørgensen, M.; Grønbaek, M.; Tolstrup, J.; Becker, U. Alcohol and cirrhosis: Dose--response or threshold effect? J. Hepatol. 2004, 41, 25–30. [Google Scholar] [CrossRef]
- Ramstedt, M. Per capita alcohol consumption and liver cirrhosis mortality in 14 European countries. Addiction 2001, 96 (Suppl. S1), S19–S33. [Google Scholar] [CrossRef]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef]
- Jamal, M.M.; Saadi, Z.; Morgan, T.R. Alcohol and hepatitis C. Dig. Dis. 2005, 23, 285–296. [Google Scholar] [CrossRef]
- Irving, H.M.; Samokhvalov, A.V.; Rehm, J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. JOP 2009, 10, 387–392. [Google Scholar] [PubMed]
- Apte, M.V.; Wilson, J.S. Alcohol-induced pancreatic injury. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 593–612. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Fuchs, S.C. The Effect of Alcohol on Blood Pressure and Hypertension. Curr. Hypertens Rep. 2021, 23, 42. [Google Scholar] [CrossRef] [PubMed]

| Protective Effects | Negative Effects |
|---|---|
| Alcohol and CVD | |
| Drinking pattern: low-moderate alcohol (♀ ≤7drinks/week, ♂ ≤14 drinks/week) + with meals + avoid binge drinking | Chronic alcohol consumption, heavy drinking |
| Effects on blood pressure, atrial fibrillation, or strokes | High blood pressure, cardiomyopathy, coronary heart disease |
| Articles: [12,13,16,17,21,22,23,24,25,26,27,28,29,30,39,40,141] | Articles: [47,48,49,50,142,143,144] |
| Alcohol and Diabetes Mellitus | |
| Low-moderate alcohol consumption | Chronic alcohol consumption, heavy drinking |
| Reduced insulin resistance, HbA1c levels and CVD risk | Disruption in glucose homeostasis, higher insulin resistance, less adherence to diabetes treatment |
| Articles: [31,99,145] | Articles: [99,146,147] |
| Alcohol and Digestive diseases | |
| Low-moderate consumption | Chronic alcohol consumption, heavy drinking |
| - | Hepatitis, chronic liver disease, cirrhosis, pancreatitis, gastritis |
| Articles: [148] | Articles: [2,4,149,150,151,152,153,154,155] |
| Alcohol and Cancer | |
| Low (1–5 g/day), moderate (12–25 g/day) | All alcohol consumption, especially heavy and chronic patterns |
| Lowest risk of cancer (even alcohol related cancers) | Higher risk of breast, oropharynx, larynx, larynx, esophagus, liver, colon and rectum cancers |
| Articles: [37,73,74] | Articles: [2,4,6,59,60,71,72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbería-Latasa, M.; Gea, A.; Martínez-González, M.A. Alcohol, Drinking Pattern, and Chronic Disease. Nutrients 2022, 14, 1954. https://doi.org/10.3390/nu14091954
Barbería-Latasa M, Gea A, Martínez-González MA. Alcohol, Drinking Pattern, and Chronic Disease. Nutrients. 2022; 14(9):1954. https://doi.org/10.3390/nu14091954
Chicago/Turabian StyleBarbería-Latasa, María, Alfredo Gea, and Miguel A. Martínez-González. 2022. "Alcohol, Drinking Pattern, and Chronic Disease" Nutrients 14, no. 9: 1954. https://doi.org/10.3390/nu14091954
APA StyleBarbería-Latasa, M., Gea, A., & Martínez-González, M. A. (2022). Alcohol, Drinking Pattern, and Chronic Disease. Nutrients, 14(9), 1954. https://doi.org/10.3390/nu14091954